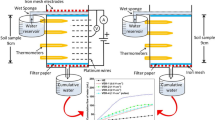
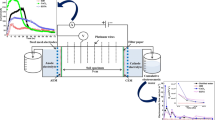
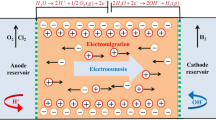
Abstract
Electrokinetic is an effective method for the extraction of contaminants from low-permeable soils. This work examines the influence of Ca+2 ions on the electroosmotic flow and the effect of electric potential, current, and pH variations on the removal of Pb2+, Na+, and Clˉ ions from artificially contaminated soil during vertical electrokinetic experiments. The DC electric field of 1 Vcm−1 was applied across the soil specimen via steel mesh electrodes for 24, 48, and 72 h of the experiment. In this work, the vertical electrokinetic cell was used to avoid the deposition of Na+ and Pb +2 ions near the soil surface after the treatment. making it even less permeable. The results show that the formation of acidic and alkaline environments in soil specimens affects the transport of ionic species by reducing the effect of electromigration and electroosmotic water flow. The enhancement of electroosmosis using Ca+2 ions as an electrolyte increased the extraction efficiency of Pb2+ (i.e., 41%) and Na+ (i.e., 82%) ions instead of the Clˉ (i.e., 69%) ions due to the high electroosmotic flow (i.e., 81 mL) from anode to the cathode. For relatively low electroosmotic flow (i.e., 19 mL), the extraction efficiency of Clˉ ions (i.e., 76%) was higher than that of Pb2+ (i.e., 27%) and Na+ (i.e., 44%) ions. The results demonstrated that the extraction efficiency of ions and energy consumption increased with treatment time and were higher during the first 24 h of the experiments.
Graphical Abstract






Similar content being viewed by others
References
Butcher K, Wick AF, Desutter T et al (2016) Soil salinity: a threat to global food security. Agron J 108:2189–2200. https://doi.org/10.2134/agronj2016.06.0368
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445. https://doi.org/10.1016/S1369-5266(03)00085-2
Munns R, Day DA, Fricke W et al (2020) Energy costs of salt tolerance in crop plants. New Phytol 225:1072–1090. https://doi.org/10.1111/nph.15864
Jones BEH, Haynes RJ (2011) Bauxite processing residue: a critical review of its formation, properties, storage, and revegetation. Crit Rev Environ Sci Technol 41:271–315. https://doi.org/10.1080/10643380902800000
Kim SO, Jeong JY, Lee WC et al (2021) Electrokinetic remediation of heavy metal-contaminated soils: performance comparison between one- and two-dimensional electrode configurations. J Soils Sediments 21:2755–2769. https://doi.org/10.1007/s11368-020-02803-z
Bessaim MM, Missoum H, Bendani K et al (2020) Effect of processing time on removal of harmful emerging salt pollutants from saline-sodic soil during electrochemical remediation. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.126688
Cameselle C, Pena A (2016) Enhanced electromigration and electro-osmosis for the remediation of an agricultural soil contaminated with multiple heavy metals. Process Saf Environ Prot 104:209–217. https://doi.org/10.1016/j.psep.2016.09.002
Alshawabkeh AN, Sheahan TC, Wu X (2004) Coupling of electrochemical and mechanical processes in soils under DC fields. Mech Mater 36:453–465. https://doi.org/10.1016/S0167-6636(03)00071-1
Cameselle C, Reddy KR (2012) Development and enhancement of electro-osmotic flow for the removal of contaminants from soils. Electrochim Acta 86:10–22. https://doi.org/10.1016/j.electacta.2012.06.121
Cameselle C (2015) Enhancement of electro-osmotic flow during the electrokinetic treatment of a contaminated soil. Electrochim Acta 181:31–38. https://doi.org/10.1016/j.electacta.2015.02.191
Lim AE, Lim CY, Lam YC (2016) Electroosmotic flow hysteresis for dissimilar anionic solutions. Anal Chem 88:8064–8073. https://doi.org/10.1021/acs.analchem.6b01536
Sumbarda-Ramos EG, Guerrero-Gutierrez OX, Murillo-Rivera B et al (2010) Electrokinetic treatment for clayed and sandy soils. J Appl Electrochem 40:1255–1261. https://doi.org/10.1007/s10800-010-0097-7
Yeung AT, Gu YY (2011) A review on techniques to enhance electrochemical remediation of contaminated soils. J Hazard Mater 195:11–29. https://doi.org/10.1016/j.jhazmat.2011.08.047
Wen D, Fu R, Li Q (2021) Removal of inorganic contaminants in soil by electrokinetic remediation technologies: a review. J Hazard Mater 401:123345. https://doi.org/10.1016/j.jhazmat.2020.123345
Li D, Huang T, Liu K (2016) Near-anode focusing phenomenon caused by the coupling effect of early precipitation and backward electromigration in electrokinetic remediation of MSWI fly ashes. Environ Technol (United Kingdom) 37:216–227. https://doi.org/10.1080/09593330.2015.1066873
Chien SC, Teng FC, Ou CY (2015) Soil improvement of electroosmosis with the chemical treatment using the suitable operation process. Acta Geotech 10:813–820. https://doi.org/10.1007/s11440-014-0319-y
Wen Y, Zhang H, Su H, Ren H (2020) Optimal tracking control for non-zero-sum games of linear discrete-time systems via off-policy reinforcement learning. Optim Control Appl Methods 41:1233–1250. https://doi.org/10.1002/oca.2597
Li G, Guo S, Li S et al (2012) Comparison of approaching and fixed anodes for avoiding the “focusing” effect during electrokinetic remediation of chromium-contaminated soil. Chem Eng J 203:231–238. https://doi.org/10.1016/j.cej.2012.07.008
Abou-Shady A (2016) Reclaiming salt-affected soils using electro-remediation technology: PCPSS evaluation. Electrochim Acta 190:511–520. https://doi.org/10.1016/j.electacta.2016.01.036
Estefan G, Sommer R, Ryan J (2013) Methods of Soil , Plant , and Water Analysis : A manual for the West Asia and North. 1–243
Peng J, Ye H, Alshawabkeh AN (2015) Soil improvement by electroosmotic grouting of saline solutions with vacuum drainage at the cathode. Appl Clay Sci 114:53–60. https://doi.org/10.1016/j.clay.2015.05.012
Ishaq M, Kamran K, Jamil Y, Sarfraz RA (2021) Electric potential and current distribution in contaminated porous building materials under electrokinetic desalination. Mater Struct Constr. https://doi.org/10.1617/s11527-021-01770-2
Beretta AN, Silbermann AV, Paladino L et al (2014) Análisis de textura del suelo con hidrómetro: modificaciones al método de bouyoucus. Cienc e Investig Agrar 41:263–271. https://doi.org/10.4067/S0718-16202014000200013
Bagarello V, Iovino M, Palazzolo E et al (2006) Field and laboratory approaches for determining sodicity effects on saturated soil hydraulic conductivity. Geoderma 130:1–13. https://doi.org/10.1016/j.geoderma.2005.01.004
Li H, Tan Y, Xie Z et al (2021) Method for measuring the saturated permeability coefficient of compacted bentonite at temperatures exceeding 100 °C. Prog Nucl Energy 141:103958. https://doi.org/10.1016/j.pnucene.2021.103958
Bensharada M, Telford R, Stern B, Gaffney V (2022) Loss on ignition vs. thermogravimetric analysis: a comparative study to determine organic matter and carbonate content in sediments. J Paleolimnol 67:191–197. https://doi.org/10.1007/s10933-021-00209-6
Zhu S, Zhu D, Wang X (2017) Removal of fluorine from red mud (bauxite residue) by electrokinetics. Electrochim Acta 242:300–306. https://doi.org/10.1016/j.electacta.2017.05.040
Kamran K, Van Soestbergen M, Huinink HP, Pel L (2012) Inhibition of electrokinetic ion transport in porous materials due to potential drops induced by electrolysis. Electrochim Acta 78:229–235. https://doi.org/10.1016/j.electacta.2012.05.123
Wada SI, Umegaki Y (2001) Major ion and electrical potential distribution in soil under electrokinetic remediation. Environ Sci Technol 35:2151–2155. https://doi.org/10.1021/es001335j
Raza A, Haidry AA, Shahzad MK et al (2022) Adsorption kinetics and photocatalytic properties of Cu2ZnSnS4@porous g-C3N4 for contaminant removal. Mater Sci Semicond Process 150:106912. https://doi.org/10.1016/j.mssp.2022.106912
Liu X, Feng B, Tian R et al (2020) Electrical double layer interactions between soil colloidal particles: polarization of water molecule and counterion. Geoderma. 380:114693. https://doi.org/10.1016/j.geoderma.2020.114693
Zulfiqar W, Iqbal MA, Butt MK (2017) Pb2+ ions mobility perturbation by iron particles during electrokinetic remediation of contaminated soil. Chemosphere 169:257–261. https://doi.org/10.1016/j.chemosphere.2016.11.083
Kim JH, Han SJ, Kim SS, Yang JW (2006) Effect of soil chemical properties on the remediation of phenanthrene-contaminated soil by electrokinetic-Fenton process. Chemosphere 63:1667–1676. https://doi.org/10.1016/j.chemosphere.2005.10.008
Ye WM, Zheng ZJ, Chen B et al (2014) Effects of pH and temperature on the swelling pressure and hydraulic conductivity of compacted GMZ01 bentonite. Appl Clay Sci 101:192–198. https://doi.org/10.1016/j.clay.2014.08.002
Zhang L, Kang T, Kang J et al (2020) Effects of electrolyte pH on the electro-osmotic characteristics in anthracite. ACS Omega 5:29257–29264. https://doi.org/10.1021/acsomega.0c04013
Moayedi H, Nazir R, Kassim KA, Huat BK (2014) Measurement of the electrokinetic properties of peats treated with chemical solutions. Meas J Int Meas Confed 49:289–295. https://doi.org/10.1016/j.measurement.2013.11.008
Ait Ahmed O, Derriche Z, Kameche M et al (2016) Electro-remediation of lead contaminated kaolinite: an electro-kinetic treatment. Chem Eng Process Process Intensif 100:37–48. https://doi.org/10.1016/j.cep.2015.12.002
Ottosen LM, Christensen IV (2012) Electrochimica Acta electrokinetic desalination of sandstones for NaCl removal—test of different clay poultices at the electrodes. Electrochim Acta 86:192–202. https://doi.org/10.1016/j.electacta.2012.06.005
Yu C, Shao Z, Hou H (2017) A functionalized metal-organic framework decorated with O-groups showing excellent performance for lead(II) removal from aqueous solution. Chem Sci 8:7611–7619. https://doi.org/10.1039/c7sc03308g
Acar YB, Alshawabkeh AN (1993) Principles of electrokinetic remediation. Environ Sci Technol 27:2638–2647. https://doi.org/10.1021/es00049a002
Shu J, Sun X, Liu R et al (2019) Enhanced electrokinetic remediation of manganese and ammonia nitrogen from electrolytic manganese residue using pulsed electric field in different enhancement agents. Ecotoxicol Environ Saf 171:523–529. https://doi.org/10.1016/j.ecoenv.2019.01.025
Author information
Authors and Affiliations
Contributions
AAH wrote the manuscript and performed the experiment; KK contributed to supervision and investigation; MI, AA, and MH contributed to review the article and edited final manuscript. All authors have read and agreed to publish the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hussain, A.A., Kamran, K., Ishaq, M. et al. Optimization of electroosmotic flow to enhance the removal of contaminants from low‑permeable soils. J Appl Electrochem 53, 1245–1258 (2023). https://doi.org/10.1007/s10800-023-01845-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01845-8