Abstract
Purpose
Conventional diagnosis of primary open angle glaucoma (POAG) needs a combination of ophthalmic examinations. An efficient assay is urgently needed for a timely POAG diagnosis. We aim to explore differential expressions of circulating microRNAs (miRNA) and provide novel miRNA biomarkers for POAG diagnosis.
Methods
A total of 180 POAG patients and 210 age-related cataract (ARC) patients were enrolled. We collected aqueous humor (AH) and plasma samples from the recruited patients. The expressions of candidate miRNAs were measured using quantitative real time polymerase chain reaction. The diagnostic ability of candidate miRNAs was analyzed by receiver operating characteristic curve.
Results
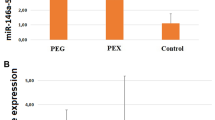
The expressions of miR-21-5p and miR-29b-3p were downregulated significantly in AH and plasma of POAG and miR-24-3p expression was significantly increased in AH and plasma of POAG, comparing with those of ARC. A three-miRNA panel was constructed by a binary logistic regression. And the panel could differentiate between POAG and ARC with an area under the curve of 0.8867 (sensitivity = 78.0%, specificity = 83.3%) in aqueous humor and 0.7547 (sensitivity = 73.8%, specificity = 81.2%) in plasma. Next, we verified the three-miRNA panel working as a potential diagnostic biomarker stable and reliable. At last, we identified related function and regulation pathways in vitro.
Conclusions
In conclusion, we built and identified a circulating three-miRNA panel as a potential diagnostic biomarker for POAG. It may be developed into an efficient assay and help improve the POAG diagnosis in the future.





Similar content being viewed by others
Data availability
The datasets are available from the corresponding author on request.
References
Stein JD, Khawaja AP, Weizer JS (2021) Glaucoma in adults-screening, diagnosis, and management: a review. JAMA 325:164–174
Weinreb RN, Leung CK, Crowston JG, Medeiros FA, Friedman DS, Wiggs JL et al (2016) Primary open-angle glaucoma. Nat Rev Dis Primers 2:16067
Fricke TR, Tahhan N, Resnikoff S, Papas E, Burnett A, Ho SM et al (2018) Global prevalence of presbyopia and vision impairment from uncorrected presbyopia: systematic review, meta-analysis, and modelling. Ophthalmology 125:1492–1499
Bussel II, Wollstein G, Schuman JS (2014) OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol 98(Suppl 2):ii15–ii19
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5:522–531
Tan C, Song M, Stamer WD, Qiao Y, Chen X, Sun X et al (2020) miR-21-5p: a viable therapeutic strategy for regulating intraocular pressure. Exp Eye Res 200:108197
Tan C, Jia F, Zhang P, Sun X, Qiao Y, Chen X et al (2021) A miRNA stabilizing polydopamine nano-platform for intraocular delivery of miR-21-5p in glaucoma therapy. J Mater Chem B 9:3335–3345
Luna C, Li G, Qiu J, Epstein DL, Gonzalez P (2011) MicroRNA-24 regulates the processing of latent TGFbeta1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J Cell Physiol 226:1407–1414
Smyth A, Callaghan B, Willoughby CE, O’Brien C (2022) The role of miR-29 family in TGF-beta driven fibrosis in glaucomatous optic neuropathy. Int J Mol Sci 23(18):10216
Li N, Cui J, Duan X, Chen H, Fan F (2012) Suppression of type I collagen expression by miR-29b via PI3K, Akt, and Sp1 pathway in human Tenon’s fibroblasts. Investig Ophthalmol Vis Sci 53:1670–1678
Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA (2011) MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat Rev Clin Oncol 8:467–477
Liu Y, Chen Y, Wang Y, Zhang X, Gao K, Chen S et al (2018) microRNA profiling in glaucoma eyes with varying degrees of optic neuropathy by using next-generation sequencing. Investig Ophthalmol Vis Sci 59:2955–2966
Hindle AG, Thoonen R, Jasien JV, Grange RMH, Amin K, Wise J et al (2019) Identification of candidate miRNA biomarkers for glaucoma. Investig Ophthalmol Vis Sci 60:134–146
Liu Y, Wang Y, Chen Y, Fang X, Wen T, Xiao M et al (2019) Discovery and validation of circulating Hsa-miR-210-3p as a potential biomarker for primary open-angle glaucoma. Investig Ophthalmol Vis Sci 60:2925–2934
Kosior-Jarecka E, Czop M, Gasinska K, Wrobel-Dudzinska D, Zalewski DP, Bogucka-Kocka A et al (2021) MicroRNAs in the aqueous humor of patients with different types of glaucoma. Graefes Arch Clin Exp Ophthalmol 259:2337–2349
Hubens WHG, Krauskopf J, Beckers HJM, Kleinjans JCS, Webers CAB, Gorgels T (2021) Small RNA sequencing of aqueous humor and plasma in patients with primary open-angle glaucoma. Investig Ophthalmol Vis Sci 62:24
Martinez B, Peplow PV (2022) MicroRNAs as biomarkers in glaucoma and potential therapeutic targets. Neural Regen Res 17:2368–2375
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF et al (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 108:5003–5008
Turchinovich A, Burwinkel B (2012) Distinct AGO1 and AGO2 associated miRNA profiles in human cells and blood plasma. RNA Biol 9:1066–1075
Ji C, Guo X (2019) The clinical potential of circulating microRNAs in obesity. Nat Rev Endocrinol 15:731–743
Lei Y, Overby DR, Read AT, Stamer WD, Ethier CR (2010) A new method for selection of angular aqueous plexus cells from porcine eyes: a model for Schlemm’s canal endothelium. Investig Ophthalmol Vis Sci 51:5744–5750
Grunkemeier GL, Jin R (2015) Net reclassification index: measuring the incremental value of adding a new risk factor to an existing risk model. Ann Thorac Surg 99:388–392
Fiedorowicz E, Cieslinska A, Kuklo P, Grzybowski A (2021) Protein biomarkers in glaucoma: a review. J Clin Med 10(22):5388
Wang Y, Hou XW, Liang G, Pan CW (2021) Metabolomics in glaucoma: a systematic review. Investig Ophthalmol Vis Sci 62:9
Zhao M, Ma P, Xie Q, Bui AD, Yonamine S, Hinterwirth A et al (2022) Biomarkers for primary open-angle glaucoma progression. Exp Eye Res 219:109025
Wang L, Yu T, Zhang X, Cai X, Sun H (2021) Network integration analysis and immune infiltration analysis reveal potential biomarkers for primary open-angle glaucoma. Front Cell Dev Biol 9:793638
Myer C, Perez J, Abdelrahman L, Mendez R, Khattri RB, Junk AK et al (2020) Differentiation of soluble aqueous humor metabolites in primary open angle glaucoma and controls. Exp Eye Res 194:108024
Leruez S, Marill A, Bresson T, de Saint MG, Buisset A, Muller J et al (2018) A metabolomics profiling of glaucoma points to mitochondrial dysfunction, senescence, and polyamines deficiency. Investig Ophthalmol Vis Sci 59:4355–4361
Kouassi Nzoughet J, Guehlouz K, Leruez S, Gohier P, Bocca C, Muller J et al (2020) A data mining metabolomics exploration of glaucoma. Metabolites 10(2):49
Wu J, Xu M, Liu W, Huang Y, Wang R, Chen W et al (2022) Glaucoma characterization by machine learning of tear metabolic fingerprinting. Small Methods 6:e2200264
Luna C, Li G, Qiu J, Epstein DL, Gonzalez P (2009) Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol Vis 15:2488–2497
Parker VA, Herrtage J, Sarkies NJ (2001) Clinical comparison of the Keeler Pulsair 3000 with Goldmann applanation tonometry. Br J Ophthalmol 85:1303–1304
Jorge J, Díaz-Rey JA, González-Méijome JM, Almeida JB, Parafita MA (2002) Clinical performance of the Reichert AT550: a new non-contact tonometer. Ophthalmic Physiol Opt J Br Coll Ophthalmic Opt Optom 22:560–564
Jorge J, González-Méijome JM, Díaz-Rey JA, Almeida JB, Ribeiro P, Parafita MA (2003) Clinical performance of non-contact tonometry by Reichert AT550 in glaucomatous patients. Ophthalmic Physiol Opt J Br Coll Ophthalmic Opt Optom 23:503–506
Acknowledgements
The clinical specimen and information of glaucoma and cataract patients described in this manuscript were obtained from the eye bank of Eye and ENT Hospital of Fudan University. We would like to thank all the staffs for their valuable contribution to this research.
Funding
This work was supported by the National Science Foundation of China (81870692, 82070959, 82271082); the Shanghai Committee of Science and Technology, China (20S31905800); Clinical Research Plan of SHDC (SHDC2020CR6029); Medical Engineering Joint Fund of Fudan University (YG202213), General project of Shanghai Natural Science Foundation (21ZR1411500, 22ZR1409900). All of above funding played the role in the design of the study and collection, analysis, and interpretation of data.
Author information
Authors and Affiliations
Contributions
Bo Qin: conceptualization, methodology, investigation, data curation, visualization and writing—original draft. Liping Li: methodology, investigation and data curation. Qingdan Xu: methodology, investigation and data curation. Yuan Lei: conceptualization, writing—review & editing, supervision and funding acquisition. Yuhong Chen: writing—review & editing, supervision and funding acquisition. All authors reviewed the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by the ethic committee of Eye & ENT Hospital, Fudan University (Ethics number: KJ2011-04) and written informed consent from all study participants was obtained.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, B., Li, Lp., Xu, Qd. et al. Identification of a circulating three-miRNA panel for the diagnosis of primary open angle glaucoma. Int Ophthalmol 44, 176 (2024). https://doi.org/10.1007/s10792-024-03100-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10792-024-03100-1