Abstract
Auraptene (AUT) is widely known to possess both antioxidant and anti-inflammatory properties. This study attempted to evaluate the protective effects of AUT in dextran sodium sulfate (DSS)-induced colitis in mice and to determine the underlying molecular mechanisms. Our results suggest that AUT substantially minimizes the severity and worsening of DSS-induced colitis in mice, indicated by the lengthening of the colon, lower disease activity index, reduced oxidation levels, and attenuated inflammatory factors. Molecular studies revealed that AUT reduces the nuclear translocation of nuclear factor-κB (NF-κB), thereby inhibiting the expression of inflammatory factors. Additionally, AUT promotes the diversity of the intestinal flora in mice with colitis by increasing the number of beneficial bacteria such as Lactobacillaceae and lowering the number of harmful bacteria. In conclusion, AUT mitigates DSS-induced colitis by maintaining the integrity of the intestinal barrier and modulating the levels of the intestinal microbial species.
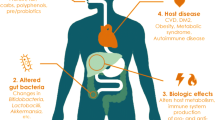
Graphical Abstract
TOC Graphic. Diagram summarizing the study findings. Auraptene mitigates dextran sulfate sodium-induced colitis in mice by regulating specific intestinal flora and repairing the intestinal barrier.










Similar content being viewed by others
Data availability
The data supporting the findings in this study will be made available upon reasonable request to the corresponding authors.
References
Wei, Y.Y., et al. 2021. Shaoyao decoction attenuates DSS-induced ulcerative colitis, macrophage and NLRP3 inflammasome activation through the MKP1/NF-κB pathway. Phytomedicine 92: 153743.
Xu, S., et al. 2021. The emerging role of ferroptosis in intestinal disease. Cell Death & Disease 12 (4): 289.
Huang, F., et al. 2022. STAT3-mediated ferroptosis is involved in ulcerative colitis. Free Radical Biology & Medicine 188: 375–385.
Salice, M., et al. 2019. A current overview of corticosteroid use in active ulcerative colitis. Expert Review of Gastroenterology & Hepatology 13 (6): 557–561.
Glover, L.E., J.S. Lee, and S.P. Colgan. 2016. Oxygen metabolism and barrier regulation in the intestinal mucosa. The Journal of Clinical Investigation 126 (10): 3680–3688.
Li, C., Y.Y. Cai, and Z.X. Yan. 2018. Brain-derived neurotrophic factor preserves intestinal mucosal barrier function and alters gut microbiota in mice. Kaohsiung Journal of Medical Sciences 34 (3): 134–141.
Ramanan, D., and K. Cadwell. 2016. Intrinsic defense mechanisms of the intestinal epithelium. Cell Host & Microbe 19 (4): 434–441.
Xu, D., et al. 2022. Orally administered ginkgolide C attenuates DSS-induced colitis by maintaining gut barrier integrity, inhibiting inflammatory responses, and regulating intestinal flora. Journal of Agriculture and Food Chemistry 70 (46): 14718–14731.
Lee, S.H. 2015. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intestinal Research 13 (1): 11–18.
Chen, W.X., L.H. Ren, and R.H. Shi. 2014. Enteric microbiota leads to new therapeutic strategies for ulcerative colitis. World Journal of Gastroenterology 20 (42): 15657–15663.
Tan, A.H., S.Y. Lim, and A.E. Lang. 2022. The microbiome-gut-brain axis in Parkinson disease - from basic research to the clinic. Nature Reviews Neurology 18 (8): 476–495.
Xavier, R.J., and D.K. Podolsky. 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448 (7152): 427–434.
Schirmer, M., et al. 2019. Microbial genes and pathways in inflammatory bowel disease. Nature Reviews Microbiology 17 (8): 497–511.
Askari, V.R., et al. 2021. Anti-oxidant and anti-inflammatory effects of auraptene on phytohemagglutinin (PHA)-induced inflammation in human lymphocytes. Pharmacological Reports 73 (1): 154–162.
Genovese, S., and F. Epifano. 2011. Auraptene: A natural biologically active compound with multiple targets. Current Drug Targets 12 (3): 381–386.
Shao, X., et al. 2020. Anti-Inflammatory and Intestinal Microbiota Modulation Properties of Jinxiang Garlic (Allium sativum L.) Polysaccharides toward Dextran Sodium Sulfate-Induced Colitis. Journal of Agricultural and Food Chemistry 68 (44): 12295–12309.
Tayarani-Najaran, Z., N. Tayarani-Najaran, and S. Eghbali. 2021. A review of auraptene as an anticancer agent. Frontiers in Pharmacology 12: 698352.
Bibak, B., et al. 2019. A review of the pharmacological and therapeutic effects of auraptene. BioFactors 45 (6): 867–879.
Mizoguchi, A. 2012. Animal models of inflammatory bowel disease. Progress in Molecular Biology and Translational Science 105: 263–320.
Park, Y.H., et al. 2015. Adequate dextran sodium sulfate-induced colitis model in mice and effective outcome measurement method. Journal of Cancer Prevention 20 (4): 260–267.
Yang, L., et al. 2021. Tanshinone I and tanshinone IIA/B attenuate LPS-induced mastitis via regulating the NF-κB. Biomedicine & Pharmacotherapy 137: 111353.
Zhang, Y., et al. 2022. Alginate oligosaccharides ameliorate DSS-induced colitis through modulation of AMPK/NF-κB pathway and intestinal microbiota. Nutrients 14(14).
Zhao, Y., et al. 2018. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunology 11 (3): 752–762.
Yap, Y.A., and E. Marino. 2018. An insight into the intestinal web of mucosal immunity, microbiota, and diet in inflammation. Frontiers in Immunology 9: 2617.
Camilleri, M., et al. 2012. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterology and Motility 24 (6): 503–512.
Günzel, D., and M. Fromm. 2012. Claudins and other tight junction proteins. Comprehensive Physiology 2 (3): 1819–1852.
Cui, L., et al. 2021. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. International Journal of Biological Macromolecules 166: 1035–1045.
Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nature Immunology 2 (8): 675–680.
Vunta, H., et al. 2007. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta 12,14-prostaglandin J2 in macrophages. Journal of Biological Chemistry 282 (25): 17964–17973.
Schottelius, A.J., and H. Dinter. 2006. Cytokines, NF-kappaB, microenvironment, intestinal inflammation and cancer. Cancer Treatment and Research 130: 67–87.
Shin, S.Y., et al. 2016. The UPR inducer DPP23 inhibits the metastatic potential of MDA-MB-231 human breast cancer cells by targeting the Akt-IKK-NF-κB-MMP-9 axis. Science and Reports 6: 34134.
Stojanov, S., A. Berlec, and B. Strukelj. 2020. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 8(11).
Dias, A.M.M., et al. 2021. Lactobacillus stress protein GroEL prevents colonic inflammation. Journal of Gastroenterology 56 (5): 442–455.
Vallianou, N., et al. 2021. Do antibiotics cause obesity through long-term alterations in the gut microbiome? A review of current evidence. Current Obesity Reports 10 (3): 244–262.
Funding
This study was supported by the Norman Bethune Program of Jilin University (Grant No. 2022B15) and the Science and Technology Development Program of Jilin Province (Grant Nos. YDZJ202301ZYTS103 and YDZJ202201ZYTS002).
Author information
Authors and Affiliations
Contributions
Conceptualization: Xuedong Fang, Tong Chen, Naizhong Jin, Zhongming Li, and Qiutao Wang. Methodology: Qiutao and Tong Chen. Formal analysis: Xuedong Fang and Naizhong Jin. Investigation: Zhongming Li, Qiutao Wang, and Tong Chen. Data curation: Tong Chen. Writing—original draft: Xuedong Fang. Writing—review and editing: Qiutao Wang.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, T., Jin, N., Zhang, Q. et al. Auraptene Mitigates Colitis Induced by Dextran Sulfate Sodium in Mice by Regulating Specific Intestinal Flora and Repairing the Intestinal Barrier. Inflammation (2024). https://doi.org/10.1007/s10753-023-01965-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-023-01965-5