Abstract
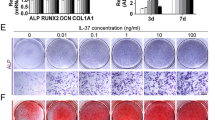
The osteogenic potential of mesenchymal stem cells (MSc) in axial spondyloarthritis (AxSpA) depends on the interplay of inflammation and multiple hormonal and local mechanical factors. In this study, MCs, derived from the adipose tissue of a healthy donor, were cultured under or without continuous mechanical load in the osteogenic differentiation medium with or without the addition of testosterone, cocktail of INF-γ/TNF-α/IL-22, or both. Real-time PCR for osteogenic transcription factors demonstrated that in the absence of INF-γ/TNF-α/IL-22, mechanical load causes significant upregulation of SPP1 (osteopontin), while the presence of the inflammatory cytokines almost completely abolishes this effect. In addition, exposure to INF-γ/TNF-α/IL-22 slightly upregulated BMP2, but suppressed the expression of ALPL, Col1A1, and SPP1, reinforcing the hypothesis that the inflammatory environment allows MSc to commit toward the IL-22-driven osteogenic differentiation but can restrict the later stages of osteogenesis. In summary, osteopontin can play a role in the pathogenesis of AxSpA, linking between mechanical load and pathological bone formation.


Similar content being viewed by others
Data Availability
RT-PCR data is available upon request.
References
Nakamura, A., A. Talukdar, S. Nakamura, E. Pathan, and N. Haroon. 2019. Bone formation in axial spondyloarthritis: is disease modification possible? Best Practice and Research in Clinical Rheumatology 33: 101491.
Van Mechelen, M., G.R. Gulino, K. de Vlam, and R. Lories. 2018. Bone disease in axial spondyloarthritis. Calcified Tissue International 102: 547–558.
Molnar, C., A. Scherer, X. Baraliakos, et al. 2018. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: Results from the Swiss Clinical Quality Management cohort. Annals of the Rheumatic Diseases 77: 63–69.
Wright, G.C., J. Kaine, and A. Deodhar. 2020. Understanding differences between men and women with axial spondyloarthritis. Seminars in Arthritis and Rheumatism 50: 687–694.
Ramiro, S., C. Stolwijk, A. van Tubergen, et al. 2015. Evolution of radiographic damage in ankylosing spondylitis: A 12 year prospective follow-up of the OASIS study. Annals of the Rheumatic Diseases 74: 52–59.
Tan, S., J. Yao, J.A. Flynn, L. Yao, and M.M. Ward. 2015. Dynamics of syndesmophyte growth in AS as measured by quantitative CT: Heterogeneity within and among vertebral disc spaces. Rheumatology (Oxford) 54: 972–980.
Pacheco-Tena, C., S.A. González-Chávez, C. Quiñonez-Flores, and R. Burgos-Vargas. 2015. Bone proliferation in ankylosing tarsitis might involve mechanical stress, and hormonal and growth factors. Journal of Rheumatology 42: 2210.
Cambré, I., D. Gaublomme, A. Burssens, et al. 2018. Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis. Nature Communications 9: 4613.
Machado, P.M., X. Baraliakos, D. van der Heijde, J. Braun, and R. Landewé. 2016. MRI vertebral corner inflammation followed by fat deposition is the strongest contributor to the development of new bone at the same vertebral corner: A multilevel longitudinal analysis in patients with ankylosing spondylitis. Annals of the Rheumatic Diseases 75: 1486–1493.
El-Zayadi, A.A., E.A. Jones, S.M. Churchman, et al. 2017. Interleukin-22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: A novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology (Oxford) 56: 488–493.
Choi, S.T., J.H. Kim, E.J. Kang, et al. 2008. Osteopontin might be involved in bone remodelling rather than in inflammation in ankylosing spondylitis. Rheumatology (Oxford) 47: 1775–1779.
Genre, F., R. López-Mejías, J.A. Miranda-Filloy, et al. 2014. Correlation between two biomarkers of atherosclerosis, osteopontin and angiopoietin-2, in non-diabetic ankylosing spondylitis patients undergoing TNF-α antagonist therapy. Clinical and Experimental Rheumatology 32: 231–236.
Li, J., Y. Cai, Z. Wang, A. Deng, and G. Yang. 2018. Polymorphisms in the osteopontin are associated with susceptibility to ankylosing spondylitis in a Han Chinese population. BioMed Research International 2018: 3458439.
Liu, J., L. Zhao, X. Yang, et al. 2022. Bone mineral density, bone metabolism-related factors, and microRNA-218 are correlated with disease activities in Chinese ankylosing spondylitis patients. Journal of Clinical Laboratory Analysis 36: e24223.
Kaaij, M.H., B. Helder, L.J.J. van Mens, M.G.H. van de Sande, D.L.P. Baeten, and S.W. Tas. 2020. Anti-IL-17A treatment reduces serum inflammatory, angiogenic and tissue remodeling biomarkers accompanied by less synovial high endothelial venules in peripheral spondyloarthritis. Science and Reports 10: 21094.
Pacheco-Tena, C., R. Pérez-Tamayo, C. Pineda, et al. 2015. Bone lineage proteins in the entheses of the midfoot in patients with spondyloarthritis. Journal of Rheumatology 42: 630–637.
Yager, N., S. Cole, A. Lledo Lara, et al. 2021. Ex vivo mass cytometry analysis reveals a profound myeloid proinflammatory signature in psoriatic arthritis synovial fluid. Annals of the Rheumatic Diseases 80: 1559–1567.
Zhang, P., Y. Wu, Q. Dai, B. Fang, and L. Jiang. 2013. p38-MAPK signaling pathway is not involved in osteogenic differentiation during early response of mesenchymal stem cells to continuous mechanical strain. Molecular and Cellular Biochemistry 378: 19–28.
Acknowledgements
We are grateful to Bonus BioGroup, Haifa, Israel, for donating us adipose tissue-derived MSc for these experiments, and to Genomics Center of Technion, Haifa, Israel, for performing real-time PCR tests for the study.
Funding
The study was partially supported by Novartis, Israel.
Author information
Authors and Affiliations
Contributions
A.C., H.H., D.A., L.S., and G.S.: design. A.C., M.B., and T.T.: laboratory experiments. A.C., T.T, S.K., and G.S.: interpretation of the results. A.C. and G.S.: preparation of the manuscript. All authors: critical review of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Osteopontin expression is upregulated by mechanical load and can be involved in the pathogenesis new bone formation in axial spondyloarthritis.
• INF-γ/TNF-α/Il-22 containing environment allows the commitment of mesenchymal stem cells to osteogenic differentiation but can impede the later stages of osteogenesis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shouval, A., Brant-Roznavi, M., Tamari, T. et al. Osteopontin Upregulation, Induced by the Continuous Mechanical Load in Adipose Tissue-Derived Mesenchymal Stem Cells, is Strongly Restricted in INF-γ/TNF-α/IL-22 Microenvironment. Inflammation 46, 2270–2275 (2023). https://doi.org/10.1007/s10753-023-01876-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01876-5