Abstract
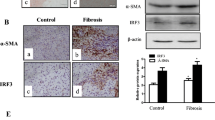
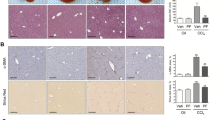
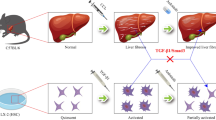
Quiescent hepatic stellate cells (qHSCs), converted to myofibroblasts, produce fibrous scars, which is an essential event during liver fibrogenesis. Clinical and experimental fibrosis undergo remarkable regression when the underlying etiological agent is removed. Some myofibroblasts revert to an inactive phenotype (iHSCs) during the regression of fibrosis. However, the mechanisms underlying HSC activation and reversal remain unclear. The present study demonstrated that the expression of lymphocyte-specific protein tyrosine kinase (LCK) was increased in fibrotic livers but decreased after spontaneous recovery in vivo and in vitro, which was correlated with the expression of α-smooth muscle actin (α-SMA) and type I collagen (COL-1). Further investigation indicated that specific knockdown of LCK by a recombination adeno-associated virus 9 (rAAV9) in C57BL/6 mice ameliorated liver fibrosis. Co-incubation of TGF-β1-induced HSC-T6 cells with LCK-siRNA inhibited cell proliferation and activation. Overexpression of LCK inhibited activated HSCs going to inactivated phenotype. Interestingly, we found that LCK may interact with suppressor of cytokine signaling 1 (SOCS1) and may influence the expression of p-JAK1 and p-STAT1/3. These data suggest that LCK may play a regulatory role in liver fibrosis by inhibiting SOCS1, indicating that LCK is a potential therapeutic target for liver fibrosis treatment.








Similar content being viewed by others
Abbreviations
- rAAV9:
-
Recombination adeno-associated viruses
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- α-SMA:
-
α-Smooth muscle actin
- CCl4 :
-
Carbon tetrachloride
- COl1α1:
-
Type I collagen
- ECM:
-
Extracellular matrix
- HA:
-
Hyaluronic acid
- HSC:
-
Hepatic stellate cell
- JAK1:
-
Janus kinase-1
- LCK:
-
Lymphocyte-specific protein tyrosine kinase
- LN:
-
Laminin
- PCIII:
-
Type 3 procollagen
- siRNA:
-
Small interfering RNA
- SOCS1:
-
Suppressor of cytokine signaling 1
- STAT1:
-
Signal transducer and activator of transcription 1
- STAT3:
-
Signal transducer and activator of transcription 3.
References
Ginès, P., A. Krag, J.G. Abraldes, E. Solà, N. Fabrellas, and P.S. Kamath. 2021. Liver cirrhosis. The Lancet 398: 1359–1376.
Berumen, J., J. Baglieri, T. Kisseleva, and K. Mekeel. 2021. Liver fibrosis: Pathophysiology and clinical implications. WIREs Mech Dis 13: e1499.
Tsuchida, T., and S.L. Friedman. 2017. Mechanisms of hepatic stellate cell activation. Nature Reviews. Gastroenterology & Hepatology 14: 397–411.
Bu, F.T., Y. Zhu, X. Chen, A. Wang, Y.F. Zhang, H.M. You, Y. Yang, Y.R. Yang, C. Huang, and J. Li. 2021. Circular RNA circPSD3 alleviates hepatic fibrogenesis by regulating the miR-92b-3p/Smad7 axis. Molecular Therapy Nucleic Acids 23: 847–862.
Higashi, T., S.L. Friedman, and Y. Hoshida. 2017. Hepatic stellate cells as key target in liver fibrosis. Advanced Drug Delivery Reviews 121: 27–42.
Calvente, C.J., M. Tameda, C.D. Johnson, H. Del Pilar, Y.C. Lin, N. Adronikou, X. De Mollerat Du, C. Jeu, J. Llorente, and A.E. Feldstein. Boyer. 2019. Neutrophils contribute to spontaneous resolution of liver inflammation and fibrosis via microRNA-223. The Journal of Clinical Investigation 129: 4091–4109.
Kisseleva, T., M. Cong, Y. Paik, D. Scholten, C. Jiang, C. Benner, K. Iwaisako, T. Moore-Morris, B. Scott, H. Tsukamoto, S.M. Evans, W. Dillmann, C.K. Glass, and D.A. Brenner. 2012. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proceedings of the National Academy of Sciences of the United States of America 109: 9448–9453.
Krizhanovsky, V., M. Yon, R.A. Dickins, S. Hearn, J. Simon, C. Miething, H. Yee, L. Zender, and S.W. Lowe. 2008. Senescence of activated stellate cells limits liver fibrosis. Cell 134: 657–667.
Kisseleva, T., and D. Brenner. 2021. Molecular and cellular mechanisms of liver fibrosis and its regression. Nature Reviews. Gastroenterology & Hepatology 18: 151–166.
Liu, X., Y. Wu, Y. Yang, W. Li, C. Huang, X. Meng, and J. Li. 2016. Role of NLRC5 in progression and reversal of hepatic fibrosis. Toxicology and Applied Pharmacology 294: 43–53.
Li, X., X.Q. Wu, T. Xu, X.F. Li, Y. Yang, W.X. Li, C. Huang, X.M. Meng, and J. Li. 2016. Role of histone deacetylases(HDACs) in progression and reversal of liver fibrosis. Toxicology and Applied Pharmacology 306: 58–68.
Wang, S., and S.L. Friedman. 2020. Hepatic fibrosis: A convergent response to liver injury that is reversible. Journal of Hepatology 73: 210–211.
Yu, H.X., Y. Yao, F.T. Bu, Y. Chen, Y.T. Wu, Y. Yang, X. Chen, Y. Zhu, Q. Wang, X.Y. Pan, X.M. Meng, C. Huang, and J. Li. 2019. Blockade of YAP alleviates hepatic fibrosis through accelerating apoptosis and reversion of activated hepatic stellate cells. Molecular Immunology 107: 29–40.
Bu, F.T., Y. Chen, H.X. Yu, X. Chen, Y. Yang, X.Y. Pan, Q. Wang, Y.T. Wu, C. Huang, X.M. Meng, and J. Li. 2018. SENP2 alleviates CCl4-induced liver fibrosis by promoting activated hepatic stellate cell apoptosis and reversion. Toxicology Letters 289: 86–98.
You, H., L. Wang, F. Bu, H. Meng, X. Pan, J. Li, Y. Zhang, A. Wang, N. Yin, C. Huang, and J. Li. 2021. The miR-455-3p/HDAC2 axis plays a pivotal role in the progression and reversal of liver fibrosis and is regulated by epigenetics. The FASEB Journal 35: e21700.
Kisseleva, T., and D.A. Brenner. 2011. Anti-fibrogenic strategies and the regression of fibrosis. Best Practice & Research Clinical Gastroenterology 25: 305–317.
Li, L., Y. Cui, J. Shen, H. Dobson, and G. Sun. 2019. Evidence for activated Lck protein tyrosine kinase as the driver of proliferation in acute myeloid leukemia cell, CTV-1. Leukemia Research 78: 12–20.
Zepecki, J.P., K.M. Snyder, M.M. Moreno, E. Fajardo, A. Fiser, J. Ness, A. Sarkar, S.A. Toms, and N. Tapinos. 2019. Regulation of human glioma cell migration, tumor growth, and stemness gene expression using a Lck targeted inhibitor. Oncogene 38: 1734–1750.
Shi, M., J.C. Cooper, and C.L. Yu. 2006. A constitutively active Lck kinase promotes cell proliferation and resistance to apoptosis through signal transducer and activator of transcription 5b activation. Molecular Cancer Research 4: 39–45.
Abraham, N., M. Carrie Miceli, J.R. Parnes, and A. Veillete. 1991. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature 350.
Wei, Q., J. Brzostek, S. Sankaran, J. Casas, L.S. Hew, J. Yap, X. Zhao, L. Wojciech, and N.R.J. Gascoigne. 2020. Lck bound to coreceptor is less active than free Lck. Proceedings of the National Academy of Sciences of the United States of America 117: 15809–15817.
Bommhardt, U., B. Schraven, and L. Simeoni. 2019. Beyond TCR signaling: emerging functions of lck in cancer and immunotherapy. International Journal of Molecular Sciences 20.
Talab, F., J.C. Allen, V. Thompson, K. Lin, and J.R. Slupsky. 2013. LCK is an important mediator of B-cell receptor signaling in chronic lymphocytic leukemia cells. Molecular Cancer Research 11: 541–554.
Betapudi, V., M. Shukla, R. Alluri, S. Merkulov, and K.R. McCrae. 2016. Novel role for p56/Lck in regulation of endothelial cell survival and angiogenesis. The FASEB Journal 30: 3515–3526.
Mas, V.R., D.G. Maluf, K.J. Archer, K. Yanek, X. Kong, L. Kulik, C.E. Freise, K.M. Olthoff, R.M. Ghobrial, P. McIver, and R. Fisher. 2009. Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma. Molecular Medicine 15: 85–94.
Wurmbach, E., Y.B. Chen, G. Khitrov, W. Zhang, S. Roayaie, M. Schwartz, I. Fiel, S. Thung, V. Mazzaferro, J. Bruix, E. Bottinger, S. Friedman, S. Waxman, and J.M. Llovet. 2007. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology 45: 938–947.
Mejias, M., J. Gallego, S. Naranjo-Suarez, M. Ramirez, N. Pell, A. Manzano, C. Suner, R. Bartrons, R. Mendez, and M. Fernandez. 2020. CPEB4 increases expression of PFKFB3 to induce glycolysis and activate mouse and human hepatic stellate cells, promoting liver fibrosis. Gastroenterology 159: 273–288.
Conboy, C.B., J.A. Yonkus, E.H. Buckarma, D.G. Mun, N.W. Werneburg, R.D. Watkins, R. Alva-Ruiz, J.L. Tomlinson, Y. Guo, J. Wang, D. O’Brien, C.E. McCabe, E. Jessen, R.P. Graham, R.C. Buijsman, D. Vu, J. de Man, S.I. Ilyas, M.J. Truty, M. Borad, A. Pandey, G.J. Gores, and R.L. Smoot. 2023. LCK inhibition downregulates YAP activity and is therapeutic in patient-derived models of cholangiocarcinoma. Journal of Hepatology 78: 142–152.
Kumar Singh, P., A. Kashyap, and O. Silakari. 2018. Exploration of the therapeutic aspects of Lck: A kinase target in inflammatory mediated pathological conditions. Biomedicine & Pharmacotherapy 108: 1565–1571.
Liu, J., Z. Guo, Y. Zhang, T. Wu, Y. Ma, W. Lai, and Z. Guo. 2020. LCK inhibitor attenuates atherosclerosis in ApoE(-/-) mice via regulating T cell differentiation and reverse cholesterol transport. Journal of Molecular and Cellular Cardiology 139: 87–97.
Zhao, H., L. Wu, Y. Zhang, S. Feng, Y. Ding, X. Deng, R. Feng, J. Li, T. Ma, and C. Huang. 2022. Betulinic acid prevents liver fibrosis by binding Lck and suppressing Lck in HSC activation and proliferation. Journal of Ethnopharmacology 296: 115459.
Venkitachalam, S., F. Y. Chueh, K. F. Leong, P. S., C. L. Yu. 2011. Suppressor of cytokine signaling 1 interacts with oncogenic lymphocyte-specific protein tyrosine kinase. Oncology Reports 25.
Cooper, J.C., M. Shi, F.Y. Chueh, S. Venkitachalam, and C.L. Yu. 2010. Enforced SOCS1 and SOCS3 expression attenuates Lck-mediated cellular transformation. International Journal of Oncology 36: 1201–1208.
Cheng, C., C. Huang, T.T. Ma, E.B. Bian, Y. He, L. Zhang, and J. Li. 2014. SOCS1 hypermethylation mediated by DNMT1 is associated with lipopolysaccharide-induced inflammatory cytokines in macrophages. Toxicology Letters 225: 488–497.
Zhu, H., H. Zhao, S. Xu, Y. Zhang, Y. Ding, J. Li, C. Huang, and T. Ma. 2021. Sennoside A alleviates inflammatory responses by inhibiting the hypermethylation of SOCS1 in CCl4-induced liver fibrosis. Pharmacological Research 174: 105926.
Mafanda, E.K., R. Kandhi, D. Bobbala, M.G.M. Khan, M. Nandi, A. Menendez, S. Ramanathan, and S. Ilangumaran. 2019. Essential role of suppressor of cytokine signaling 1 (SOCS1) in hepatocytes and macrophages in the regulation of liver fibrosis. Cytokine 124: 154501.
Kandhi, R., D. Bobbala, M. Yeganeh, M. Mayhue, A. Menendez, and S. Ilangumaran. 2016. Negative regulation of the hepatic fibrogenic response by suppressor of cytokine signaling 1. Cytokine 82: 58–69.
Chim, C.S., T.K. Fung, W.C. Cheung, R. Liang, and Y.L. Kwong. 2004. SOCS1 and SHP1 hypermethylation in multiple myeloma: Implications for epigenetic activation of the Jak/STAT pathway. Blood 103: 4630–4635.
Marti-Rodrigo, A., F. Alegre, A.B. Moragrega, F. Garcia-Garcia, P. Marti-Rodrigo, A. Fernandez-Iglesias, J. Gracia-Sancho, N. Apostolova, J.V. Esplugues, and A. Blas-Garcia. 2020. Rilpivirine attenuates liver fibrosis through selective STAT1-mediated apoptosis in hepatic stellate cells. Gut 69: 920–932.
Luan, J., J. Fu, D. Wang, C. Jiao, X. Cui, C. Chen, D. Liu, Y. Zhang, Y. Wang, P.S.T. Yuen, J.B. Kopp, J. Pi, and H. Zhou. 2020. miR-150-based RNA interference attenuates tubulointerstitial fibrosis through the SOCS1/JAK/STAT pathway in vivo and in vitro. Molecular Therapy Nucleic Acids 22: 871–884.
Feng, Y., J. Ren, Y. Gui, W. Wei, B. Shu, Q. Lu, X. Xue, X. Sun, W. He, J. Yang, and C. Dai. 2018. Wnt/beta-catenin-promoted macrophage alternative activation contributes to kidney fibrosis. Journal of the American Society of Nephrology 29: 182–193.
Liang, Y.B., H. Tang, Z.B. Chen, L.J. Zeng, J.G. Wu, W. Yang, Z.Y. Li, and Z.F. Ma. 2017. Downregulated SOCS1 expression activates the JAK1/STAT1 pathway and promotes polarization of macrophages into M1 type. Molecular Medicine Reports 16: 6405–6411.
Acknowledgements
The authors thank the Center for Scientific Research of Anhui Medical University for valuable help in our experiment. This work was supported by funding from the National Science Foundation of China (82070628) and the University Synergy Innovation Program of Anhui Province (GXXT-2019-045, GXXT-2020-063, GXXT-2020-025). The authors thank the Center for Scientific Research of Anhui Medical University for valuable help in our experiment.
Author information
Authors and Affiliations
Contributions
Huizi Zhao and Hong Zhu wrote the manuscript text. Yuan Zhang and Yuhao Ding prepared figures and collected samples. Rui Feng, Jun Li, Taotao Ma, and Cheng Huang designed the experiment.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, H., Zhu, H., Zhang, Y. et al. Lymphocyte-Specific Protein Tyrosine Kinase Contributes to Spontaneous Regression of Liver Fibrosis may by Interacting with Suppressor of Cytokine Signaling 1. Inflammation 46, 1653–1669 (2023). https://doi.org/10.1007/s10753-023-01831-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01831-4