Abstract
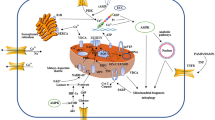
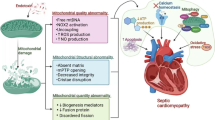
Sepsis is a lethal syndrome with multiple organ failure caused by an inappropriate host response to infection. Cardiac dysfunction is one of the important complications of sepsis, termed sepsis-induced myocardial dysfunction (SIMD), which is characterized by systolic and diastolic dysfunction of both sides of the heart. Mechanisms that contribute to SIMD include an excessive inflammatory response, altered circulatory, microvascular status, nitric oxide (NO) synthesis impairment, endothelial dysfunction, disorders of calcium regulation, cardiac autophagy anomaly, autonomic nervous system dysregulation, metabolic reprogramming, and mitochondrial dysfunction. The role of mitochondrial dysfunction, which is characterized by structural abnormalities, increased oxidative stress, abnormal opening of the mitochondrial permeability transition pore (mPTP), mitochondrial uncoupling, and disordered quality control systems, has been gaining increasing attention as a central player in the pathophysiology of SIMD. The disruption of homeostasis within the organism induced by mitochondrial dysfunction may also be an important aspect of SIMD development. In addition, an emerging therapy strategy targeting mitochondria, namely, metabolic resuscitation, seems promising. The current review briefly introduces the mechanism of SIMD, highlights how mitochondrial dysfunction contributes to SIMD, and discusses the role of metabolic resuscitation in the treatment of SIMD.

Similar content being viewed by others
Abbreviations
- PAMPs:
-
pathogen-associated molecular patterns
- DAMPs:
-
damage-associated molecular patterns
- O2 :
-
dioxygen
- Ca2+ :
-
calcium bivalent
- TLR:
-
toll-like receptor
- ATP:
-
adenosine triphosphate
- ROS:
-
reactive oxygen species
- RNS:
-
reactive nitrogen species
- LPS:
-
lipopolysaccharide
- CLP:
-
cecal ligation and puncture
- mtDNA:
-
mitochondrial DNA
- mtTFA/TFAM:
-
mitochondrial transcription factor A
- mPTP:
-
mitochondrial permeability transition pore
- AMP:
-
adenosine monophosphate
- MAMs:
-
mitochondrial-associated membranes
- AMPK:
-
AMP-activated protein kinase
- IL:
-
interleukin
- HMGB1:
-
high mobility group protein B1
- PGC1α:
-
peroxisome proliferator-activated receptor gamma coactivator-1 α
- PGC1β:
-
peroxisome proliferator-activated receptor gamma coactivator-1 β
- ICU:
-
intensive care unit
- RCT:
-
randomized controlled trial
- TNFα:
-
tumor necrosis factor
- SOFA:
-
sequential organ failure assessment
- CO:
-
carbon monoxide
- NO:
-
nitric oxide
- H2S:
-
hydrogen sulfide
References
Singer, M., C.S. Deutschman, C.W. Seymour, M. Shankar-Hari, D. Annane, M. Bauer, R. Bellomo, G.R. Bernard, J.D. Chiche, C.M. Coopersmith, R.S. Hotchkiss, M.M. Levy, J.C. Marshall, G.S. Martin, S.M. Opal, G.D. Rubenfeld, T. van der Poll, J.L. Vincent, and D.C. Angus. 2016. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 315 (8): 801–810.
Reinhart, K., R. Daniels, N. Kissoon, F.R. Machado, R.D. Schachter, and S. Finfer. 2017. Recognizing sepsis as a global health priority - a WHO resolution. The New England Journal of Medicine 377 (5): 414–417.
Court, O., A. Kumar, J.E. Parrillo, and A. Kumar. 2002. Clinical review: myocardial depression in sepsis and septic shock. Critical care (London, England)6 (6): 500–508.
Liu, Y.C., M.M. Yu, S.T. Shou, and Y.F. Chai. 2017. Sepsis-induced cardiomyopathy: mechanisms and treatments. Frontiers in Immunology 8: 1021.
Beesley, S.J., G. Weber, T. Sarge, S. Nikravan, C.K. Grissom, M.J. Lanspa, S. Shahul, and S.M. Brown. 2018. Septic cardiomyopathy. Critical Care Medicine 46 (4): 625–634.
Martin, L., M. Derwall, S. Al Zoubi, E. Zechendorf, D.A. Reuter, C. Thiemermann, and T. Schuerholz. 2019. The septic heart: current understanding of molecular mechanisms and clinical implications. Chest 155 (2): 427–437.
Fenton, K.E., and M.M. Parker. 2016. Cardiac function and dysfunction in sepsis. Clinics in Chest Medicine 37 (2): 289–298.
Singer, M. 2014. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 5 (1): 66–72.
Takasu, O., J.P. Gaut, E. Watanabe, To K, R.E. Fagley, B. Sato, S. Jarman, I.R. Efimov, D.L. Janks, A. Srivastava, et al. 2013. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. American Journal of Respiratory and Critical Care Medicine 187 (5): 509–517.
Vanasco, V., T. Saez, N.D. Magnani, L. Pereyra, T. Marchini, A. Corach, M.I. Vaccaro, D. Corach, P. Evelson, and S. Alvarez. 2014. Cardiac mitochondrial biogenesis in endotoxemia is not accompanied by mitochondrial function recovery. Free Radical Biology & Medicine 77: 1–9.
Supinski, G.S., and L.A. Callahan. 2006. Polyethylene glycol-superoxide dismutase prevents endotoxin-induced cardiac dysfunction. American Journal of Respiratory and Critical Care Medicine 173 (11): 1240–1247.
Lowes, D.A., B.M. Thottakam, N.R. Webster, M.P. Murphy, and H.F. Galley. 2008. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radical Biology & Medicine 45 (11): 1559–1565.
Zang, Q.S., H. Sadek, D.L. Maass, B. Martinez, L. Ma, J.A. Kilgore, N.S. Williams, D.E. Frantz, J.G. Wigginton, F.E. Nwariaku, S.E. Wolf, and J.P. Minei. 2012. Specific inhibition of mitochondrial oxidative stress suppresses inflammation and improves cardiac function in a rat pneumonia-related sepsis model. American Journal of Physiology Heart and Circulatory Physiology 302 (9): H1847–H1859.
Vanasco, V., M.C. Cimolai, P. Evelson, and S. Alvarez. 2008. The oxidative stress and the mitochondrial dysfunction caused by endotoxemia are prevented by alpha-lipoic acid. Free Radical Research 42 (9): 815–823.
Suliman, H.B., K.E. Welty-Wolf, M. Carraway, L. Tatro, and C.A. Piantadosi. 2004. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovascular Research 64 (2): 279–288.
Joshi, M.S., M.W. Julian, J.E. Huff, J.A. Bauer, Y. Xia, and E.D. Crouser. 2006. Calcineurin regulates myocardial function during acute endotoxemia. American Journal of Respiratory and Critical Care Medicine 173 (9): 999–1007.
Larche, J., S. Lancel, S.M. Hassoun, R. Favory, B. Decoster, P. Marchetti, C. Chopin, and R. Neviere. 2006. Inhibition of mitochondrial permeability transition prevents sepsis-induced myocardial dysfunction and mortality. Journal of the American College of Cardiology 48 (2): 377–385.
Pan, P., H. Zhang, L. Su, X. Wang, and D. Liu. 2018. Melatonin balance the autophagy and apoptosis by regulating UCP2 in the LPS-induced cardiomyopathy. Molecules (Basel, Switzerland) 23 (3).
Xu, C., C. Yi, H. Wang, I.C. Bruce, and Q. Xia. 2012. Mitochondrial nitric oxide synthase participates in septic shock myocardial depression by nitric oxide overproduction and mitochondrial permeability transition pore opening. Shock (Augusta, Ga) 37 (1): 110–115.
Petronilli, V., D. Penzo, L. Scorrano, P. Bernardi, and F. Di Lisa. 2001. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. The Journal of Biological Chemistry 276 (15): 12030–12034.
Wang, X., D. Liu, W. Chai, Y. Long, L. Su, and R. Yang. 2015. The role of uncoupling protein 2 during myocardial dysfunction in a canine model of endotoxin shock. Shock (Augusta, Ga) 43 (3): 292–297.
Zheng, G., J. Lyu, S. Liu, J. Huang, C. Liu, D. Xiang, M. Xie, and Q. Zeng. 2015. Silencing of uncoupling protein 2 by small interfering RNA aggravates mitochondrial dysfunction in cardiomyocytes under septic conditions. International Journal of Molecular Medicine 35 (6): 1525–1536.
Sánchez-Villamil, J.P., V. D'Annunzio, P. Finocchietto, S. Holod, I. Rebagliati, H. Pérez, J.G. Peralta, R.J. Gelpi, J.J. Poderoso, and M.C. Carreras. 2016. Cardiac-specific overexpression of thioredoxin 1 attenuates mitochondrial and myocardial dysfunction in septic mice. The international journal of biochemistry & cell biology 81 (Pt B): 323–334.
Gonzalez, A.S., M.E. Elguero, P. Finocchietto, S. Holod, L. Romorini, S.G. Miriuka, J.G. Peralta, J.J. Poderoso, and M.C. Carreras. 2014. Abnormal mitochondrial fusion-fission balance contributes to the progression of experimental sepsis. Free Radical Research 48 (7): 769–783.
Preau, S., F. Delguste, Y. Yu, I. Remy-Jouet, V. Richard, F. Saulnier, E. Boulanger, and R. Neviere. 2016. Endotoxemia engages the RhoA kinase pathway to impair cardiac function by altering cytoskeleton, mitochondrial fission, and autophagy. Antioxidants & Redox Signaling 24 (10): 529–542.
Hickson-Bick, D.L., C. Jones, and L.M. Buja. 2008. Stimulation of mitochondrial biogenesis and autophagy by lipopolysaccharide in the neonatal rat cardiomyocyte protects against programmed cell death. Journal of Molecular and Cellular Cardiology 44 (2): 411–418.
Piquereau, J., R. Godin, S. Deschênes, V.L. Bessi, M. Mofarrahi, S.N. Hussain, and Y. Burelle. 2013. Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy 9 (11): 1837–1851.
Kim, M.J., S.H. Bae, J.C. Ryu, Y. Kwon, J.H. Oh, J. Kwon, J.S. Moon, K. Kim, A. Miyawaki, M.G. Lee, et al. 2016. SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages. Autophagy 12 (8): 1272–1291.
Carré, J.E., J.C. Orban, L. Re, K. Felsmann, W. Iffert, M. Bauer, H.B. Suliman, C.A. Piantadosi, T.M. Mayhew, P. Breen, M. Stotz, and M. Singer. 2010. Survival in critical illness is associated with early activation of mitochondrial biogenesis. American Journal of Respiratory and Critical Care Medicine 182 (6): 745–751.
Russell, L.K., C.M. Mansfield, J.J. Lehman, A. Kovacs, M. Courtois, J.E. Saffitz, D.M. Medeiros, M.L. Valencik, J.A. McDonald, and D.P. Kelly. 2004. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circulation Research 94 (4): 525–533.
Drosatos, K., R.S. Khan, C.M. Trent, H. Jiang, N.H. Son, W.S. Blaner, S. Homma, P.C. Schulze, and I.J. Goldberg. 2013. Peroxisome proliferator-activated receptor-γ activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circulation Heart failure 6 (3): 550–562.
Schilling, J., L. Lai, N. Sambandam, C.E. Dey, T.C. Leone, and D.P. Kelly. 2011. Toll-like receptor-mediated inflammatory signaling reprograms cardiac energy metabolism by repressing peroxisome proliferator-activated receptor γ coactivator-1 signaling. Circulation Heart failure 4 (4): 474–482.
Solomon, M.A., R. Correa, H.R. Alexander, L.A. Koev, J.P. Cobb, D.K. Kim, W.C. Roberts, Z.M. Quezado, T.D. Scholz, and R.E. Cunnion. 1994. Myocardial energy metabolism and morphology in a canine model of sepsis. The American Journal of Physiology 266 (2 Pt 2): H757–H768.
Zang, Q., D.L. Maass, S.J. Tsai, and J.W. Horton. 2007. Cardiac mitochondrial damage and inflammation responses in sepsis. Surgical Infections 8 (1): 41–54.
Neri, M., I. Riezzo, C. Pomara, S. Schiavone, and E. Turillazzi. 2016. Oxidative-nitrosative stress and myocardial dysfunctions in sepsis: evidence from the literature and postmortem observations. Mediators of Inflammation 2016: 3423450.
Bolaños, J.P., S.J. Heales, S. Peuchen, J.E. Barker, J.M. Land, and J.B. Clark. 1996. Nitric oxide-mediated mitochondrial damage: a potential neuroprotective role for glutathione. Free Radical Biology & Medicine 21 (7): 995–1001.
Supinski, G.S., M.P. Murphy, and L.A. Callahan. 2009. MitoQ administration prevents endotoxin-induced cardiac dysfunction. American journal of physiology Regulatory, integrative and comparative physiology 297 (4): R1095–R1102.
Halestrap, A.P., G.P. McStay, and S.J. Clarke. The permeability transition pore complex: another view. Biochimie84 (2–3): 153–166.
Halestrap, A.P. 2009. What is the mitochondrial permeability transition pore? Journal of Molecular and Cellular Cardiology 46 (6): 821–831.
Hüser, J., and L.A. Blatter. 1999. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. The Biochemical Journal 343 (Pt 2): 311–317.
Petronilli, V., G. Miotto, M. Canton, M. Brini, R. Colonna, P. Bernardi, and F. Di Lisa. 1999. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophysical Journal 76 (2): 725–734.
Divakaruni, A.S., and M.D. Brand. 2011. The regulation and physiology of mitochondrial proton leak. Physiology (Bethesda, Md) 26 (3): 192–205.
Ruiz-Ramírez, A., O. López-Acosta, M.A. Barrios-Maya, and M. El-Hafidi. 2016. Cell death and heart failure in obesity: role of uncoupling proteins. Oxidative Medicine and Cellular Longevity 2016: 9340654.
Youle, R.J., and A.M. van der Bliek. 2012. Mitochondrial fission, fusion, and stress. Science (New York, NY) 337 (6098): 1062–1065.
Kubli, D.A., and Å.B. Gustafsson. 2012. Mitochondria and mitophagy: the yin and yang of cell death control. Circulation Research 111 (9): 1208–1221.
Dorn, G.W., and R.N. Kitsis. 2015. The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble. Circulation Research 116 (1): 167–182.
Kelly, D.P., and R.C. Scarpulla. 2004. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes & Development 18 (4): 357–368.
Wenz, T. 2013. Regulation of mitochondrial biogenesis and PGC-1α under cellular stress. Mitochondrion 13 (2): 134–142.
Yao, X., D. Carlson, Y. Sun, L. Ma, S.E. Wolf, J.P. Minei, and Q.S. Zang. 2015. Mitochondrial ROS induces cardiac inflammation via a pathway through mtDNA damage in a pneumonia-related sepsis model. PLoS One 10 (10): e0139416.
Chouchani, E.T., L. Kazak, and B.M. Spiegelman. 2017. Mitochondrial reactive oxygen species and adipose tissue thermogenesis: Bridging physiology and mechanisms. The Journal of Biological Chemistry 292 (41): 16810–16816.
Ortega, S.P., E.T. Chouchani, and S. Boudina. 2017. Stress turns on the heat: regulation of mitochondrial biogenesis and UCP1 by ROS in adipocytes. Adipocyte 6 (1): 56–61.
Yu, X.X., J.L. Barger, B.B. Boyer, M.D. Brand, G. Pan, and S.H. Adams. 2000. Impact of endotoxin on UCP homolog mRNA abundance, thermoregulation, and mitochondrial proton leak kinetics. American Journal of Physiology Endocrinology and Metabolism 279 (2): E433–E446.
Chen, H.W., C. Hsu, T.S. Lu, S.J. Wang, and R.C. Yang. 2003. Heat shock pretreatment prevents cardiac mitochondrial dysfunction during sepsis. Shock (Augusta, Ga) 20 (3): 274–279.
Miller, W.L. 2013. Steroid hormone synthesis in mitochondria. Molecular and Cellular Endocrinology 379 (1–2): 62–73.
Maechler, P. 2013. Mitochondrial function and insulin secretion. Molecular and Cellular Endocrinology 379 (1–2): 12–18.
Chow, J., J. Rahman, J.C. Achermann, M.T. Dattani, and S. Rahman. 2017. Mitochondrial disease and endocrine dysfunction. Nature Reviews Endocrinology 13 (2): 92–104.
Sharma, A.C., H.B. Bosmann, S.J. Motew, K.H. Hales, D.B. Hales, and J.L. Ferguson. 1996. Steroid hormone alterations following induction of chronic intraperitoneal sepsis in male rats. Shock (Augusta, Ga) 6 (2): 150–154.
Fourrier, F., A. Jallot, L. Leclerc, M. Jourdain, A. Racadot, J.L. Chagnon, A. Rime, and C. Chopin. 1994. Sex steroid hormones in circulatory shock, sepsis syndrome, and septic shock. Circulatory Shock 43 (4): 171–178.
Borkowski, J., A. Siemiatkowski, M. Jedynak, S.L. Czaban, and S. Wołczyński. 2003. Serum levels of luteinizing hormone, testosterone and prolactin in patients with septic shock. Przeglad lekarski 60 (11): 706–709.
Marx, C., S. Petros, S.R. Bornstein, M. Weise, M. Wendt, M. Menschikowski, L. Engelmann, and G. Höffken. 2003. Adrenocortical hormones in survivors and nonsurvivors of severe sepsis: diverse time course of dehydroepiandrosterone, dehydroepiandrosterone-sulfate, and cortisol. Critical Care Medicine 31 (5): 1382–1388.
Zhang, Q., G. Dong, X. Zhao, M. Wang, and C.S. Li. 2014. Prognostic significance of hypothalamic-pituitary-adrenal axis hormones in early sepsis: a study performed in the emergency department. Intensive Care Medicine 40 (10): 1499–1508.
Giorgi, C., S. Marchi, and P. Pinton. 2018. The machineries, regulation and cellular functions of mitochondrial calcium. Nature Reviews Molecular Cell Biology 19 (11): 713–730.
Bravo-Sagua, R., V. Parra, C. López-Crisosto, P. Díaz, A.F. Quest, and S. Lavandero. 2017. Calcium transport and signaling in mitochondria. Comprehensive Physiology 7 (2): 623–634.
Berridge, M.J., P. Lipp, and M.D. Bootman. 2000. The versatility and universality of calcium signalling. Nature Reviews Molecular Cell Biology 1 (1): 11–21.
Hassoun, S.M., X. Marechal, D. Montaigne, Y. Bouazza, B. Decoster, S. Lancel, and R. Neviere. 2008. Prevention of endotoxin-induced sarcoplasmic reticulum calcium leak improves mitochondrial and myocardial dysfunction. Critical Care Medicine 36 (9): 2590–2596.
Joseph, L.C., D. Kokkinaki, M.C. Valenti, G.J. Kim, E. Barca, D. Tomar, N.E. Hoffman, P. Subramanyam, H.M. Colecraft, M. Hirano, et al. 2017. Inhibition of NADPH oxidase 2 (NOX2) prevents sepsis-induced cardiomyopathy by improving calcium handling and mitochondrial function. JCI insight 2 (17).
Pinto, B.B., A. Dyson, M. Umbrello, J.E. Carré, C. Ritter, I. Clatworthy, M.R. Duchen, and M. Singer. 2017. Improved survival in a long-term rat model of sepsis is associated with reduced mitochondrial calcium uptake despite increased energetic demand. Critical Care Medicine 45 (8): e840–e848.
Chandel, N.S. 2014. Mitochondria as signaling organelles. BMC Biology 12: 34.
Chandel, N.S. 2015. Evolution of mitochondria as signaling organelles. Cell Metabolism 22 (2): 204–206.
Chan, S.H., K.L. Wu, L.L. Wang, and J.Y. Chan. 2005. Nitric oxide- and superoxide-dependent mitochondrial signaling in endotoxin-induced apoptosis in the rostral ventrolateral medulla of rats. Free Radical Biology & Medicine 39 (5): 603–618.
Emre, Y., C. Hurtaud, T. Nübel, F. Criscuolo, D. Ricquier, and A.M. Cassard-Doulcier. 2007. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. The Biochemical Journal 402 (2): 271–278.
Neviere, R., F. Delguste, A. Durand, J. Inamo, E. Boulanger, and S. Preau. 2016. Abnormal mitochondrial cAMP/PKA signaling is involved in sepsis-induced mitochondrial and myocardial dysfunction. International Journal of Molecular Sciences17 (12).
Zang, Q.S., B. Martinez, X. Yao, D.L. Maass, L. Ma, S.E. Wolf, and J.P. Minei. 2012. Sepsis-induced cardiac mitochondrial dysfunction involves altered mitochondrial-localization of tyrosine kinase Src and tyrosine phosphatase SHP2. PLoS One 7 (8): e43424.
Quirós, P.M., A. Mottis, and J. Auwerx. 2016. Mitonuclear communication in homeostasis and stress. Nature Reviews Molecular Cell Biology 17 (4): 213–226.
Haynes, C.M., C.J. Fiorese, and Y.F. Lin. 2013. Evaluating and responding to mitochondrial dysfunction: the mitochondrial unfolded-protein response and beyond. Trends in Cell Biology 23 (7): 311–318.
Huang, L.J., H.P. Dong, I.C. Chuang, M.S. Liu, and R.C. Yang. 2012. Attenuation of mitochondrial unfolded protein response is associated with hepatic dysfunction in septic rats. Shock (Augusta, Ga), 38 (6): 642–648.
West, A.P., W. Khoury-Hanold, M. Staron, M.C. Tal, C.M. Pineda, S.M. Lang, M. Bestwick, B.A. Duguay, N. Raimundo, D.A. MacDuff, et al. 2015. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520 (7548): 553–557.
Breda, C.N.S., G.G. Davanzo, P.J. Basso, N.O. Saraiva Câmara, and P.M.M. Moraes-Vieira. 2019. Mitochondria as central hub of the immune system. Redox Biology 26: 101255.
Vakifahmetoglu-Norberg, H., A.T. Ouchida, and E. Norberg. 2017. The role of mitochondria in metabolism and cell death. Biochemical and Biophysical Research Communications 482 (3): 426–431.
Gurung, P., J.R. Lukens, and T.D. Kanneganti. 2015. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends in Molecular Medicine 21 (3): 193–201.
Xu, X., Q. Liu, S. He, J. Zhao, N. Wang, X. Han, and Y. Guo. 2018. Qiang-Xin 1 formula prevents Sepsis-induced apoptosis in murine cardiomyocytes by suppressing endoplasmic reticulum- and mitochondria-associated pathways. Frontiers in Pharmacology 9: 818.
Hotchkiss, R.S., S.B. Osmon, K.C. Chang, T.H. Wagner, C.M. Coopersmith, and I.E. Karl. 2005. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. Journal of immunology (Baltimore, Md : 1950) 174 (8): 5110–5118.
Wang, P., Y. Hu, D. Yao, and Y. Li. 2018. Omi/HtrA2 regulates a mitochondria-dependent apoptotic pathway in a murine model of septic encephalopathy. Cellular Physiology and Biochemistry : International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 49 (6): 2163–2173.
Chen, G., X. Li, M. Huang, X. Zhou, Y. Li, X. Mao, and J. Bai. 2017. The role of thioredoxin-1 in suppression sepsis through inhibiting mitochondrial-induced apoptosis in spleen. Shock (Augusta, Ga) 47 (6): 753–758.
Leite, H.P., and L.F. de Lima. 2016. Metabolic resuscitation in sepsis: a necessary step beyond the hemodynamic? Journal of Thoracic Disease 8 (7): E552–E557.
Ding, W., Y. Shen, Q. Li, S. Jiang, and H. Shen. 2018. Therapeutic mild hypothermia improves early outcomes in rats subjected to severe sepsis. Life Sciences 199: 1–9.
Léon, K., K. Pichavant-Rafini, H. Ollivier, V. Monbet, and E. L'Her. 2015. Does induction time of mild hypothermia influence the survival duration of septic rats? Therapeutic Hypothermia and Temperature Management 5 (2): 85–88.
Schwarzl, M., S. Seiler, M. Wallner, D. von Lewinski, S. Huber, H. Maechler, P. Steendijk, S. Zelzer, M. Truschnig-Wilders, B. Obermayer-Pietsch, A. Lueger, B.M. Pieske, and H. Post. 2013. Mild hypothermia attenuates circulatory and pulmonary dysfunction during experimental endotoxemia. Critical Care Medicine 41 (12): e401–e410.
Itenov, T.S., M.E. Johansen, M. Bestle, K. Thormar, L. Hein, L. Gyldensted, A. Lindhardt, H. Christensen, S. Estrup, H.P. Pedersen, et al. 2018. Induced hypothermia in patients with septic shock and respiratory failure (CASS): A randomised, controlled, open-label trial. The Lancet Respiratory Medicine 6 (3): 183–192.
Zhang, Z., L. Chen, and H. Ni. 2015. Antipyretic therapy in critically ill patients with sepsis: an interaction with body temperature. PLoS One 10 (3): e0121919.
López-Lluch, G., and P. Navas. 2016. Calorie restriction as an intervention in ageing. The Journal of Physiology 594 (8): 2043–2060.
Starr, M.E., A.M. Steele, D.A. Cohen, and H. Saito. 2016. Short-term dietary restriction rescues mice from lethal abdominal sepsis and endotoxemia and reduces the inflammatory/coagulant potential of adipose tissue. Critical Care Medicine 44 (7): e509–e519.
Hasegawa, A., H. Iwasaka, S. Hagiwara, N. Asai, T. Nishida, and T. Noguchi. 2012. Alternate day calorie restriction improves systemic inflammation in a mouse model of sepsis induced by cecal ligation and puncture. The Journal of Surgical Research 174 (1): 136–141.
Arabi, Y.M., A.S. Aldawood, S.H. Haddad, H.M. Al-Dorzi, H.M. Tamim, G. Jones, S. Mehta, L. McIntyre, O. Solaiman, M.H. Sakkijha, et al. 2015. Permissive underfeeding or standard enteral feeding in critically ill adults. The New England Journal of Medicine 372 (25): 2398–2408.
Donnino, M.W., L.W. Andersen, M. Chase, K.M. Berg, M. Tidswell, T. Giberson, R. Wolfe, A. Moskowitz, H. Smithline, L. Ngo, M.N. Cocchi, and Center for Resuscitation Science Research Group. 2016. Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Critical Care Medicine 44 (2): 360–367.
Donnino, M.W., E. Carney, M.N. Cocchi, I. Barbash, M. Chase, N. Joyce, P.P. Chou, and L. Ngo. 2010. Thiamine deficiency in critically ill patients with sepsis. Journal of Critical Care 25 (4): 576–581.
Costa, N.A., A.L. Gut, Dorna M. de Souza, J.A. Pimentel, S.M. Cozzolino, P.S. Azevedo, A.A. Fernandes, L.A. Zornoff, S.A. de Paiva, and M.F. Minicucci. 2014. Serum thiamine concentration and oxidative stress as predictors of mortality in patients with septic shock. Journal of Critical Care 29 (2): 249–252.
Nuzzo, E., K.M. Berg, L.W. Andersen, J. Balkema, S. Montissol, M.N. Cocchi, X. Liu, and M.W. Donnino. 2015. Pyruvate dehydrogenase activity is decreased in the peripheral blood mononuclear cells of patients with Sepsis. A prospective observational trial. Annals of the American Thoracic Society 12 (11): 1662–1666.
McCall, C.E., M. Zabalawi, T. Liu, A. Martin, D.L. Long, N.L. Buechler, Arts RJW, M. Netea, B.K. Yoza, P.W. Stacpoole, et al. 2018. Pyruvate dehydrogenase complex stimulation promotes immunometabolic homeostasis and sepsis survival. JCI insight 3 (15).
Woolum, J.A., E.L. Abner, A. Kelly, M.L. Thompson Bastin, P.E. Morris, and A.H. Flannery. 2018. Effect of thiamine administration on lactate clearance and mortality in patients with septic shock. Critical Care Medicine 46 (11): 1747–1752.
Kim, J., L. Arnaout, and D. Remick. 2019. Hydrocortisone, ascorbic acid and thiamine (HAT) therapy decreases oxidative stress, improves cardiovascular function and improves survival in murine Sepsis. Shock (Augusta, Ga).
Marik, P.E., V. Khangoora, R. Rivera, M.H. Hooper, and J. Catravas. 2017. Hydrocortisone, vitamin C, and thiamine for the treatment of severe Sepsis and septic shock: a retrospective before-after study. Chest 151 (6): 1229–1238.
Litwak, J.J., N. Cho, H.B. Nguyen, K. Moussavi, and T. Bushell. 2019. Vitamin C, hydrocortisone, and thiamine for the treatment of severe sepsis and septic shock: a retrospective analysis of real-world application. Journal of clinical medicine 8 (4).
Deng, S., Y. Ai, H. Gong, Q. Feng, X. Li, C. Chen, Z. Liu, Y. Wang, Q. Peng, and L. Zhang. 2018. Mitochondrial dynamics and protective effects of a mitochondrial division inhibitor, Mdivi-1, in lipopolysaccharide-induced brain damage. Biochemical and Biophysical Research Communications 496 (3): 865–871.
Fredriksson, K., I. Tjäder, P. Keller, N. Petrovic, B. Ahlman, C. Schéele, J. Wernerman, J.A. Timmons, and O. Rooyackers. 2008. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS One 3 (11): e3686.
Cooper, C.E., and G.C. Brown. 2008. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. Journal of Bioenergetics and Biomembranes 40 (5): 533–539.
Zegdi, R., D. Perrin, M. Burdin, R. Boiteau, and A. Tenaillon. 2002. Increased endogenous carbon monoxide production in severe sepsis. Intensive Care Medicine 28 (6): 793–796.
Li, L., M. Bhatia, Y.Z. Zhu, Y.C. Zhu, R.D. Ramnath, Z.J. Wang, F.B. Anuar, M. Whiteman, M. Salto-Tellez, and P.K. Moore. 2005. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology 19 (9): 1196–1198.
Ahmad, A., D. Gerö, G. Olah, and C. Szabo. 2016. Effect of endotoxemia in mice genetically deficient in cystathionine-γ-lyase, cystathionine-β-synthase or 3-mercaptopyruvate sulfurtransferase. International Journal of Molecular Medicine 38 (6): 1683–1692.
Yu, J., J. Shi, D. Wang, S. Dong, Y. Zhang, M. Wang, L. Gong, Q. Fu, and D. Liu. 2016. Heme oxygenase-1/carbon monoxide-regulated mitochondrial dynamic equilibrium contributes to the attenuation of endotoxin-induced acute lung injury in rats and in lipopolysaccharide-activated macrophages. Anesthesiology 125 (6): 1190–1201.
Zhang, H.X., J.M. Du, Z.N. Ding, X.Y. Zhu, L. Jiang, and Y.J. Liu. 2017. Hydrogen sulfide prevents diaphragm weakness in cecal ligation puncture-induced sepsis by preservation of mitochondrial function. American Journal of Translational Research 9 (7): 3270–3281.
Wagner, F., K. Wagner, S. Weber, B. Stahl, M.W. Knöferl, M. Huber-Lang, D.H. Seitz, P. Asfar, E. Calzia, U. Senftleben, et al. 2011. Inflammatory effects of hypothermia and inhaled H2S during resuscitated, hyperdynamic murine septic shock. Shock (Augusta, Ga) 35 (4): 396–402.
Ferlito, M., Q. Wang, W.B. Fulton, P.M. Colombani, L. Marchionni, K. Fox-Talbot, N. Paolocci, and C. Steenbergen. 2014. Hydrogen sulfide [corrected] increases survival during sepsis: protective effect of CHOP inhibition. Journal of immunology (Baltimore, Md : 1950) 192 (4): 1806–1814.
Liu, J., J. Li, P. Tian, B. Guli, G. Weng, L. Li, and Q. Cheng. 2019. HS attenuates sepsis-induced cardiac dysfunction via a PI3K/Akt-dependent mechanism. Experimental and Therapeutic Medicine 17 (5): 4064–4072.
Zhang, H., S.M. Moochhala, and M. Bhatia. 2008. Endogenous hydrogen sulfide regulates inflammatory response by activating the ERK pathway in polymicrobial sepsis. Journal of immunology (Baltimore, Md : 1950) 181 (6): 4320–4331.
Otterbein, L.E., F.H. Bach, J. Alam, M. Soares, H. Tao Lu, M. Wysk, R.J. Davis, R.A. Flavell, and A.M. Choi. 2000. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nature Medicine 6 (4): 422–428.
Mazzola, S., M. Forni, M. Albertini, M.L. Bacci, A. Zannoni, F. Gentilini, M. Lavitrano, F.H. Bach, L.E. Otterbein, and M.G. Clement. 2005. Carbon monoxide pretreatment prevents respiratory derangement and ameliorates hyperacute endotoxic shock in pigs. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology 19 (14): 2045–2047.
Mitchell, L.A., M.M. Channell, C.M. Royer, S.W. Ryter, A.M. Choi, and J.D. McDonald. 2010. Evaluation of inhaled carbon monoxide as an anti-inflammatory therapy in a nonhuman primate model of lung inflammation. American journal of physiology Lung cellular and molecular physiology 299 (6): L891–L897.
Unuma, K., T. Aki, S. Nagano, R. Watanabe, and K. Uemura. 2018. The down-regulation of cardiac contractile proteins underlies myocardial depression during sepsis and is mitigated by carbon monoxide. Biochemical and Biophysical Research Communications 495 (2): 1668–1674.
Zhang, W., A. Tao, T. Lan, G. Cepinskas, R. Kao, C.M. Martin, and T. Rui. 2017. Carbon monoxide releasing molecule-3 improves myocardial function in mice with sepsis by inhibiting NLRP3 inflammasome activation in cardiac fibroblasts. Basic Research in Cardiology 112 (2): 16.
Lancel, S., S.M. Hassoun, R. Favory, B. Decoster, R. Motterlini, and R. Neviere. 2009. Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. The Journal of Pharmacology and Experimental Therapeutics 329 (2): 641–648.
Wang, P., J. Huang, Y. Li, R. Chang, H. Wu, J. Lin, and Z. Huang. 2015. Exogenous carbon monoxide decreases sepsis-induced acute kidney injury and inhibits NLRP3 inflammasome activation in rats. International Journal of Molecular Sciences 16 (9): 20595–20608.
Shi, J., J.B. Yu, W. Liu, D. Wang, Y. Zhang, L.R. Gong, S.A. Dong, and D.Q. Liu. 2016. Carbon monoxide alleviates lipopolysaccharide-induced oxidative stress injury through suppressing the expression of Fis1 in NR8383 cells. Experimental Cell Research 349 (1): 162–167.
Mayr, F.B., A. Spiel, J. Leitner, C. Marsik, P. Germann, R. Ullrich, O. Wagner, and B. Jilma. 2005. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. American Journal of Respiratory and Critical Care Medicine 171 (4): 354–360.
Marik, P.E. 2018. Vitamin C for the treatment of sepsis: the scientific rationale. Pharmacology & Therapeutics 189: 63–70.
Galley, H.F., M.J. Davies, and N.R. Webster. 1996. Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading. Free Radical Biology & Medicine 20 (1): 139–143.
Borrelli, E., P. Roux-Lombard, G.E. Grau, E. Girardin, B. Ricou, J. Dayer, and P.M. Suter. 1996. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Critical Care Medicine 24 (3): 392–397.
Fowler, A.A., A.A. Syed, S. Knowlson, R. Sculthorpe, D. Farthing, C. DeWilde, C.A. Farthing, T.L. Larus, E. Martin, D.F. Brophy, et al. 2014. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. Journal of Translational Medicine 12: 32.
Zabet, M.H., M. Mohammadi, M. Ramezani, and H. Khalili. Effect of high-dose ascorbic acid on vasopressor's requirement in septic shock. Journal of research in pharmacy practice 2016, 5 (2).
Fowler, A.A., J.D. Truwit, R.D. Hite, P.E. Morris, C. DeWilde, A. Priday, B. Fisher, L.R. Thacker, R. Natarajan, D.F. Brophy, et al. 2019. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. Jama 322 (13): 1261–1270.
Fujii, T., N. Luethi, P.J. Young, D.R. Frei, G.M. Eastwood, C.J. French, A.M. Deane, Y. Shehabi, L.A. Hajjar, G. Oliveira, et al. 2020. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: The VITAMINS randomized clinical trial. Jama 323 (5): 423–431.
Prauchner, C.A. 2017. Oxidative stress in sepsis: pathophysiological implications justifying antioxidant co-therapy. Burns : journal of the International Society for Burn Injuries 43 (3): 471–485.
Acknowledgments
We thank Accdon for its linguistic assistance during the preparation of this manuscript.
Funding
Dr. Zhaocai Zhang received funding from National Natural Science Foundation of China (No. 81772110). Dr. Yinchuan Xu received research grants from National Natural Science Foundation of China (No. 81971860 and No. 81500876); Natural Science Foundation of Zhejiang Province, China (No. LQ16H020003).
Author information
Authors and Affiliations
Contributions
Dr. Yao Lin and Dr. Yinchuan Xu performed literature searches, wrote the article, and designed the tables and figures. Dr. Yinchuan Xu and Dr. Zhaocai Zhang designed and revised the review.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 392 kb)
Rights and permissions
About this article
Cite this article
Lin, Y., Xu, Y. & Zhang, Z. Sepsis-Induced Myocardial Dysfunction (SIMD): the Pathophysiological Mechanisms and Therapeutic Strategies Targeting Mitochondria. Inflammation 43, 1184–1200 (2020). https://doi.org/10.1007/s10753-020-01233-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01233-w