Abstract
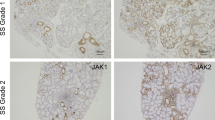
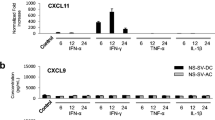
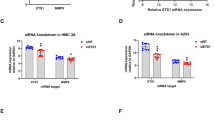
Gene expression profiling of lip salivary gland (LSG) has shown that C-X-C motif chemokine 10 (CXCL10) and matrix metalloproteinase 9 (MMP9) expression is upregulated in primary Sjögren’s syndrome (pSS) patients. Although CXCL10 and MMP-9 are both associated with pSS pathogenesis, the potential relationship between these two factors has not been investigated. In this study, we used LSG sections from pSS patients and human salivary gland cell lines to investigate the relationship between CXCL10 and MMP-9. Immunofluorescence analyses revealed that CXCL10 and MMP-9 were co-expressed in the LSG of pSS patients, particularly in expanded ductal cells. Furthermore, RT-qPCR analyses on human salivary gland ductal NS-SV-DC cells confirmed that CXCL10 expression was induced by interferon (IFN)-γ, whereas that of MMP9 was stimulated by IFN-α, tumor necrosis factor-α, and interleukin-1β. Remarkably, MMP-9 inhibition in IFN-γ-stimulated NS-SV-DC cells significantly decreased CXCL10 mRNA and secreted protein levels. Further analyses established that MMP-9 inhibition in IFN-γ-stimulated NS-SV-DC cells decreased STAT1 phosphorylation and hence suppressed IFN-γ signaling. Collectively, these results suggest that in addition to its reported role in the destruction of acinar structures, MMP-9 is involved in the IFN-γ-induced production of CXCL10 in pSS lesions. We believe that our findings open the door to the development of novel treatments for pSS, based on the modulation of MMP-9 activity.






Similar content being viewed by others
References
Alspaugh, M.A., and K. Whaley. 1981. Sjögren’s syndrome. In Textbook of Rheumatology, ed. W.N. Kelley, E.D. Harris, S. Ruddy, and C.B. Sledge, 971–999. Philadelphia: Saunders (imprint).
Vivino, F.B., V.Y. Bunya, G. Massaro-Giordano, C.R. Johr, S.L. Giattino, A. Schorpion, B. Shafer, A. Peck, K. Sivils, A. Rasmussen, J.A. Chiorini, J. He, and J.L. Ambrus Jr. 2019. Sjogren’s syndrome: An update on disease pathogenesis, clinical manifestations and treatment. Clinical Immunology 203: 81–121.
Daniel, T.E. 1984. Labial salivary gland biopsy in Sjögren’s syndrome. Assessment as a diagnostic criterion in 362 suspected cases. Arthritis and Rheumatism 27: 147–156.
Christodoulou, M.I., E.K. Kapsogeorgou, and H.M. Moutsopoulos. 2010. Characteristics of the minor salivary gland infiltrates in Sjögren’s syndrome. Journal of Autoimmunity 34: 400–407.
Kamiński, B. 2019. Laryngological manifestations of Sjögren’s syndrome. Reumatologia 57: 37–44.
Lee, Y.J., R.H. Scofield, J.Y. Hyon, P.Y. Yun, H.J. Lee, E.Y. Lee, E.B. Lee, and Y.W. Song. 2010. Salivary chemokine levels in patients with primary Sjögren’s syndrome. Rheumatology (Oxford) 49: 1747–1752.
Ogawa, N., L. Ping, L. Zhenjun, Y. Takada, and S. Sugai. 2002. Involvement of the interferon-γ-induced T cell-attracting chemokines, interferon-γ-inducible 10-kd protein (CXCL10) and monokine induced by interferon-γ (CXCL9), in the salivary gland lesions of patients with Sjögren’s syndrome. Arthritis and Rheumatism 46: 2730–2741.
Aota, K., K. Kani, T. Yamanoi, K.I. Nakashiro, N. Ishimaru, and M. Azuma. 2018. Distinct regulation of CXCL10 production by cytokines in human salivary gland ductal and acinar cells. Inflammation 41: 1172–1181.
Aota, K., T. Yamanoi, K. Kani, K.I. Nakashiro, N. Ishimaru, and M. Azuma. 2018. Inverse correlation between the number of CXCR3+ macrophages and the severity of inflammatory lesions in Sjögren's syndrome salivary glands: A pilot study. Journal of Oral Pathology and Medicine 47: 710–718.
Luster, A.D., J.C. Unkeless, and J.V. Ravetch. 1985. γ-Interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 315: 672–676.
García-López, M.A., F. Sánchez-Madrid, J.M. Rodríguez-Frade, M. Mellado, A. Acevedo, M.I. García, J.P. Albar, C. Martínez-A, and M. Marazuela. 2001. CXCR3 chemokine receptor distribution in normal and inflamed tissues: Expression on activated lymphocytes, endothelial cells, and dendritic cells. Laboratory Investigation 81: 409–418.
Groom, J.R., and A.D. Luster. 2011. CXCR3 ligands: Redundant, collaborative and antagonistic functions. Immunology and Cell Biology 89: 207–215.
Hartl, D., S. Krauss-Etschmann, B. Koller, P.L. Hordijk, T.W. Kuijpers, F. Hoffmann, A. Hector, E. Eber, V. Marcos, I. Bittmann, O. Eickelberg, M. Griese, and D. Roos. 2008. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. The Journal of Immunology 181: 8053–8067.
Tomita, K., B.L. Freeman, S.F. Bronk, N.K. LeBrasseur, T.A. White, P. Hirsova, and S.H. Ibrahim. 2016. CXCL10-mediates macrophage, but not other innate immune cells-associated inflammation in murine nonalcoholic steatohepatitis. Scientific Reports 6: 28786.
Hjelmervik, T.O., K. Petersen, I. Jonassen, R. Jonsson, and A.I. Bolstad. 2005. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjögren’s syndrome patients from healthy control subjects. Arthritis and Rheumatism 52: 1534–1544.
Szabo, K.A., R.J. Ablin, and G. Singh. 2004. Matrix metalloproteinases and the immune response. Clinical and Applied Immunology Reviews 4: 295–319.
Bernfield, M.R., S.D. Banerjee, and R.H. Cohn. 1972. Dependence of salivary epithelial morphology and branching morphogenesis upon acid mucopolysaccharide-protein (proteoglycan) at the epithelial surface. Journal of Cell Biology 52: 674–689.
Patel, V.N., and M.P. Hoffman. 2014. Salivary gland development: A template for regeneration. Seminars in Cell and Developmental Biology 25-26: 52–60.
Pérez, P., J.M. Anaya, S. Aguilera, U. Urzúa, D. Munroe, C. Molina, M.A. Hermoso, J.M. Cherry, C. Alliende, N. Olea, E. Ruiz-Narváez, and M.J. González. 2009. Gene expression and chromosomal location for susceptibility to Sjögren’s syndrome. Journal of Autoimmunity 33: 99–108.
Konttinen, Y.T., S. Halinen, R. Hanemaaijer, T. Sorsa, J. Hietanen, A. Ceponis, J.W. Xu, R. Manthorpe, J. Whittington, A. Larsson, T. Salo, L. Kjeldsen, U.H. Stenman, and A.Z. Eisen. 1998. Matrix metalloproteinase (MMP)-9 type IV collagenase/gelatinase implicated in the pathogenesis of Sjögren’s syndrome. Matrix Biology 17: 335–347.
Azuma, M., K. Aota, T. Tamatani, K. Motegi, T. Yamashita, K. Harada, Y. Hayashi, and M. Sato. 2000. Suppression of tumor necrosis factor α-induced matrix metalloproteinase 9 production by the introduction of a super-repressor form of inhibitor of nuclear factor κBα complementary DNA into immortalized human salivary gland acinar cells: Prevention of the destruction of the acinar structure in Sjögren's syndrome salivary glands. Arthritis and Rheumatism 43: 1756–1767.
Song, J., C. Wu, X. Zhang, and L.M. Sorokin. 2013. In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1β-induced peritonitis. The Journal of Immunology 190: 401–410.
Munakata, S., Y. Tashiro, C. Nishida, A. Sato, H. Komiyama, H. Shimazu, D. Dhahri, Y. Salama, S. Eiamboonsert, K. Takeda, H. Yagita, Y. Tsuda, Y. Okada, H. Nakauchi, K. Sakamoto, B. Heissig, and K. Hattori. 2015. Inhibition of plasmin protects against colitis in mice by suppressing matrix metalloproteinase 9-mediated cytokine release from myeloid cells. Gastroenterology 148: 565–578.
Fujibayashi, T., S. Sugai, N. Miyasaka, Y. Hayashi, and K. Tsubota. 2004. Revised Japanese criteria for Sjögren's syndrome (1999): Availability and validity. Modern Rheumatology 14: 425–434.
Shiboski, S.C., C.H. Shiboski, L.A. Criswell, A.N. Baer, S. Challacombe, H. Lanfranchi, M. Schiødt, H. Umehara, F. Vivino, Y. Zhao, Y. Dong, D. Greenspan, A.M. Heidenreich, P. Helin, B. Kirkham, K. Kitagawa, G. Larkin, M. Li, T. Lietman, J. Lindegaard, N. McNamara, K. Sack, P. Shirlaw, S. Sugai, C. Vollenweider, J. Whitcher, A. Wu, S. Zhang, W. Zhang, J.S. Greenspan, and T.E. Daniels for the Sjögren’s International Collaborative Clinical Alliance (SICCA) Research Groups. 2012. American College of Rheumatology classification criteria for Sjögren's syndrome: A data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care and Research 64: 475–487.
Azuma, M., T. Tamatani, Y. Kasai, and M. Sato. 1993. Immortalization of normal human salivary gland cells with duct-, myoepithelial-, acinar-, or squamous phenotype by transfection with SV40 ori- mutant deoxyribonucleic acid. Laboratory Investigation 69: 24–42.
Bach, E.A., M. Aguet, and R.D. Schreiber. 1997. The IFNγ receptor: A paradigm for cytokine receptor signaling. Annual Review of Immunology 15: 563–591.
Van Lint, P., and C. Libert. 2007. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. Journal of Leukocyte Biology 82: 1375–1381.
Riani, M., S. Le Jan, J. Plée, A. Durlach, R. Le Naour, G. Haegeman, P. Bernard, and F. Antonicelli. 2017. Bullous pemphigoid outcome is associated with CXCL10-induced matrix metalloproteinase 9 secretion from monocytes and neutrophils but not lymphocytes. The Journal of Allergy and Clinical Immunology 139: 863–872.
Ma, Z., H. Qin, and E.N. Benveniste. 2001. Transcriptional suppression of matrix metalloproteinase-9 gene expression by IFN-γ and IFN-β: Critical role of STAT-1α. The Journal of Immunology 167: 5150–5159.
Hu, X., and L.B. Ivashkiv. 2009. Cross-regulation of signaling pathways by interferon-γ: Implications for immune responses and autoimmune diseases. Immunity 31: 539–550.
Proost, P., E. Schutyser, P. Menten, S. Struyf, A. Wuyts, G. Opdenakker, M. Detheux, M. Parmentier, C. Durinx, A.M. Lambeir, J. Neyts, S. Liekens, P.C. Maudgal, A. Billiau, and J. Van Damme. 2001. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood 98: 3554–3561.
Mitsias, D.I., E.K. Kapsogeorgou, and H.M. Moutsopoulos. 2006. The role of epithelial cells in the initiation and perpetuation of autoimmune lesions: Lessons from Sjogren’s syndrome (autoimmune epithelitis). Lupus 15: 255–261.
Funding
This work was supported by the Grants-in-Aid for Scientific Research program from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (No. 19 K10311).
Author information
Authors and Affiliations
Contributions
KA and MA contributed to the study conception and design. Material preparation, data collection, and analysis were performed by KA, SO, TY, KK, and YM. The first draft of the manuscript was written by KA, and all authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Tokushima University Hospital (no. 2802) and with the 1964 Helsinki declaration and its later amendments.
Informed Consent
Written informed consent was obtained from all individual participants included in the study, and this process was documented by the Institutional Review Board of Tokushima University Hospital. The informed consent procedure was approved by the Ethics Committee of Tokushima University Hospital.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aota, K., Ono, S., Yamanoi, T. et al. MMP-9 Inhibition Suppresses Interferon-γ-Induced CXCL10 Production in Human Salivary Gland Ductal Cells. Inflammation 42, 2148–2158 (2019). https://doi.org/10.1007/s10753-019-01079-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01079-x