Abstract
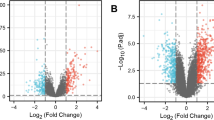
In the present study, we aimed to explore the time course pathological changes after burn injury. The time course microarray data of burn injury from the Gene Expression Omnibus (GEO) was further analyzed through bioinformatics analysis. The differential expression genes (DEGs) were identified in the early-stage vs. control groups, middle-stage vs. control groups, and early-stage vs. middle-stage groups after burn injury, followed by pathway enrichment analysis. Gene modules associated with burn injury progression were identified through weighted gene co-expression network analysis (WGCNA), and hub genes were identified via network topology analysis. There were a total of 745 DEGs in the early vs. control group, 1104 DEGs in mid vs. control, and 61 DEGs in early vs. mid group. The significant pathways enriched by DEGs in the middle stage were also enriched by DEGs in the early stage. Immunodeficiency was a significant pathway specific for the DEGs in the early stage. There were 19 overlapped genes, such as myeloperoxidase, transcobalamin, and interferon-induced protein with tetratricopeptide repeats 1, among DEGs in early vs. control, middle vs. control, and early vs. middle groups. WGCNA identified three gene modules that were significantly associated with burn injury progression. Furthermore, we identified several gene modules and biological processes that might be associated with burn injury progression, and such results may be beneficial in understanding the underlying mechanisms and developing novel drugs.




Similar content being viewed by others
References
Hunt, John L., Brett D. Arnoldo, and Gary F. Purdue. 2012. Chapter 4—prevention of burn injuries.
Haagsma, J.A., N. Graetz, I. Bolliger, M. Naghavi, H. Higashi, E.C. Mullany, S.F. Abera, J.P. Abraham, K. Adofo, and U. Alsharif. 2016. The global burden of injury: incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Injury Prevention 22 (1): 3–18.
Gupta, J.L., L.K. Makhija, and S.P. Bajaj. 2010. National programme for prevention of burn injuries. Indian Journal of Plastic Surgery Official Publication of the Association of Plastic Surgeons of India 43 (Suppl): S6–S10.
Choi, Stephen B, and A. Adam Cwinn. 2010. Rosen’s emergency medicine—concepts and clinical practice.
Alexander, J.W. 1990. Mechanism of immunologic suppression in burn injury. Journal of Trauma 30 (12 Suppl): 70–75.
Teodorczyk-Injeyan, J.A., B.G. Sparkes, G.B. Mills, W.J. Peters, and R.E. Falk. 1986. Impairment of T cell activation in burn patients: a possible mechanism of thermal injury-induced immunosuppression. Clinical and Experimental Immunology 65 (3): 570–581.
Wolfe, R.R., R.D. Goodenough, J.F. Burke, and M.H. Wolfe. 1983. Response of protein and urea kinetics in burn patients to different levels of protein intake. Annals of Surgery 197 (2): 163–171.
Saito, H., O. Trocki, Alexander Jw, R. Kopcha, T. Heyd, and Joffe Sn. 1987. The effect of route of nutrient administration on the nutritional state, catabolic hormone secretion, and gut mucosal integrity after burn injury. Journal of Parenteral & Enteral Nutrition 11 (1): 1–7.
Holmes, C.J., J.K. Plichta, R.L. Gamelli, and K.A. Radek. 2016. Burn injury alters epidermal cholinergic mediators and increases HMGB1 and caspase 3 in autologous donor skin and burn margin. Shock 47 (2): 175.
Plichta, J.K., C.J. Holmes, R.L. Gamelli, and K.A. Radek. 2017. Local burn injury promotes defects in the epidermal lipid and antimicrobial peptide barriers in human autograft skin and burn margin: Implications for burn wound healing and graft survival. Journal of Burn Care & Research 38 (1): e212.
Zhou, B., W. Xu, D. Herndon, R. Tompkins, R. Davis, W. Xiao, W.H. Wong, M. Toner, and H.S. Warren. 2010. Analysis of factorial time-course microarrays with application to a clinical study of burn injury. Proceedings of the National Academy of Sciences of the United States of America 107 (22): 9923–9928.
Benjamini, Yoav, and Yosef Hochberg. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society 57 (1): 289–300.
Moss, N.M., D.B. Gough, A.L. Jordan, J.T. Grbic, J.J. Wood, M.L. Rodrick, and J.A. Mannick. 1988. Temporal correlation of impaired immune response after thermal injury with susceptibility to infection in a murine model. Surgery 104 (5): 882–887.
Zhao, Jing, Jing Liu, James Denney, Chen Li, Fang Li, Fen Chang, Mingyou Chen, and Deling Yin. 2015. TLR2 involved in naive CD4+ T cells rescues stress-induced immune suppression by regulating Th1/Th2 and Th17. Neuroimmunomodulation 22 (5): 328–336.
Takagi, K., F. Suzuki, R.E. Barrow, S.E. Wolf, and D.N. Herndon. 1998. Recombinant human growth hormone modulates Th1 and Th2 cytokine response in burned mice. Annals of Surgery 228 (1): 106–111.
Rani, M., Q. Zhang, and M.G. Schwacha. 2014. Burn wound gammadelta T-cells support a Th2 and Th17 immune response. Journal of Burn Care & Research 35 (1): 46–53. https://doi.org/10.1097/01.bcr.0000440705.91099.cc.
Ozveri, E.S., A. Bozkurt, G. Haklar, S. Cetinel, S. Arbak, C. Yeğen, and B.C. Yeğen. 2001. Estrogens ameliorate remote organ inflammation induced by burn injury in rats. Inflammation research : official journal of the European Histamine Research Society ... [et al.] 50 (12): 585–591.
Zhang, Jing, Selena Wei Shan Sio, Shabbir Moochhala, and Madhav Bhatia. 2010. Role of hydrogen sulfide in severe burn injury–induced inflammation in mice. Molecular Medicine 16 (9–10): 417–424.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wu, D., Zhou, M., Li, L. et al. The Time Course Pathological Changes After Burn Injury. Inflammation 41, 1864–1872 (2018). https://doi.org/10.1007/s10753-018-0829-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0829-0