Abstract
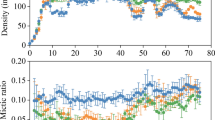
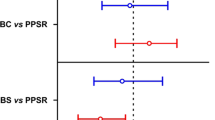
To test the hypotheses that multigenerational predation risk favors clones with longer postero-lateral spines and a larger body, and that fitness consequences of selection depend on both tested fitness variables and selection intensity, we expose replicate Brachionus dorcas populations in low (LK) and high (HK) concentrations of Asplanchna kairomones. Over 60 days (23 asexual generations) of selection, LK-selected populations showed the highest equilibrium population densities, and median mictic ratios and postero-lateral spine lengths, and HK-selected populations showed the lowest equilibrium population densities, the highest mictic ratios, and the longest postero-lateral spines, among all the control and treatment populations. In the absence of Asplanchna kairomones, and compared with populations with the control selection history, populations with an LK selection history showed increased growth rates/intrinsic rates of population increase and net reproductive rates, but decreased mictic ratios, indicating three fitness gains and a fitness cost; and those with an HK selection history showed increased life expectancy at hatching and mictic ratios, but decreased growth rates, indicating two fitness gains and a fitness cost. Asplanchna kairomones might increase the heritability of higher equilibrium densities or mictic ratios, and longer postero-lateral spines, and thus enable the prey to survive predation.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Aránguiz-Acuña, A., R. Ramos-Jiliberto, N. Sarma, S. S. S. Sarma, R. O. Bustamante & V. Toledo, 2010. Benefits, costs and reactivity of inducible defences: an experimental test with rotifers. Freshwater Biology 55: 2114–2122.
Becks, L. & A. F. Agrawal, 2010. Higher rates of sex evolve in spatially heterogeneous environments. Nature 468: 89–92.
Becks, L. & A. F. Agrawal, 2012. The evolution of sex is favored during adaptation to new environments. PLoS Biology 10: e1001317.
Becks, L. & A. F. Agrawal, 2013. Higher rates of sex evolve under K-selection. Journal of Evolutionary Biology 26: 900–905.
Ciros-Pérez, J., M. J. Carmona & M. Serra, 2002. Resource competition and patterns of sexual reproduction in sympatric sibling rotifer species. Oecologia 131: 35–42.
Declerck, A. J. S. & S. Papakostas, 2017. Monogonont rotifers as model systems for the study of micro-evolutionary adaptation and its eco-evolutionary implications. Hydrobiologia 796: 131–144.
Declerck, S. A. J., A. R. Malo, S. Diehl, D. Waasdorp, K. D. Lemmen, K. Proios & S. Papakostas, 2015. Rapid adaptation of herbivore consumers to nutrient limitation: eco-evolutionary feedbacks to population demography and resource control. Ecology Letters 18: 553–562.
Dumont, H. J. & S. S. S. Sarma, 1995. Demography and population growth of Asplanchna girodi (Rotifera) as a function of prey (Anuraeopsis fissa) density. Hydrobiologia 306: 97–107.
Freeman, J. C., L. B. Smith, J. J. Silva, Y. Fan, H. Sun & J. G. Scott, 2021. Fitness studies of insecticide resistant strains: lessons learned and future directions. Pest Management Science. https://doi.org/10.1002/ps.6306.
Fussmann, G. F., S. P. Ellner & N. G. Hairston, 2003. Evolution as a critical component of plankton dynamics. Proceedings of the Royal Society b: Biological Sciences 270: 1015–1022.
Gilbert, J. J., 1963. Mictic female production in the rotifer Brachionus calyciflorus. Journal of Experimental Zoology 153: 113–124.
Gilbert, J. J., 2013. The cost of predator-induced morphological defense in rotifers: experimental studies and synthesis. Journal of Plankton Research 35: 461–472.
Gilbert, J. J., 2014. Morphological and behavioral responses of a rotifer to the predator Asplanchna. Journal of Plankton Research 36: 1576–1584.
Gilbert, J. J., 2017. Non-genetic polymorphisms in rotifers: environmental and endogenous controls, development, and features for predictable or unpredictable environments. Biological Reviews 92: 964–992.
Gilbert, J. J., 2019. Attachment behavior in the rotifer Brachionus rubens: induction by Asplanchna and effect on sexual reproduction. Hydrobiologia 844: 9–20.
Hochmuth, D. J., L. De Meester, M. S. C. Pereira, R. C. Janssen & A. C. K. De Schamphelaere, 2015. Rapid adaptation of a Daphnia magna population to metal stress is associated with heterozygote excess. Environmental Science & Technology 49: 9298–9307.
Jiang, S., Y. Bai, F. Sun, L.-L. Ge & Y.-L. Xi, 2021. Divergent responses in morphology and life history to an Asplanchna kairomone between attached and free-swimming Brachionus dorca. Hydrobiologia 848: 1925–1937.
Lemmen, K. D., L. Zhou, S. Papakostas & S. A. J. Declerck, 2023. An experimental test of the growth rate hypothesis as a predictive framework for microevolutionary adaptation. Ecology 104: e3853.
Li, S.-H., H. Zhu, Y.-Z. Xia, M.-J. Yu, K.-S. Liu, Z.-Y. Ye & Y.-X. Chen, 1959. The mass culture of unicellular green algae. Acta Hydrobiologica Sinica 4: 462–472 (in Chinese).
Lotka, A. J., 1913. A natural population norm. Journal of Washington Academy of Sciences 3: 241–248.
Pan, L., Y.-L. Xi, J. Gu, S. Jiang, H. Zhu & B.-X. Zhang, 2017. Interactive effects of algal level and predator density (Asplanchna sieboldii) on the life-history strategy and morphology of Brachionus calyciflorus. Journal of Experimental Zoology Part a: Ecological and Integrative Physiology 327: 523–531.
Pan, L., Y.-L. Xi, J. Gu, S. Jiang, H. Zhu & B.-X. Zhang, 2018. Asplanchna-kairomone induces life history shifts in Brachionus angularis (Rotifera). Annales de Limnologie- International Journal of Limnology 54: 13.
Peacor, S. D., K. L. Pangle, L. Schiesari & E. E. Werner, 2012. Scaling-up anti-predator phenotypic responses of prey: impacts over multiple generations in a complex aquatic community. Proceedings of the Royal Society B 279: 122–128.
Peña-Aguado, F., J. Morales-Ventura, S. Nandini & S. S. S. Sarma, 2008. Influence of vertebrate and invertebrate infochemicals on the population and epizoic tendency of Brachionus rubens (Ehrenberg) (Rotifera: Brachionidae). Allelopathy Journal 22: 123–130.
Pianka, E. R., 1988. Evolutionary Ecology, 3rd ed. Harper & Row, New York.
Poole, R. W., 1974. An Introduction to Quantitative Ecology. McGraw-Hill, New York.
R Core Team, 2014. R: A Language and Environment for Statistical Computing. http://www.R-project.org. Accessed 18 September 2014.
Sarma, S. S. S., R. A. L. Resendiz & S. Nandini, 2011. Morphometric and demographic responses of brachionid prey (Brachionus calyciflorus Pallas and Plationus macracanthus (Daday)) in the presence of different densities of the predator Asplanchna brightwellii (Rotifera: Asplanchnidae). Hydrobiologia 662: 179–187.
Scheuerl, T. & C.-P. Stelzer, 2013. Patterns and dynamics of rapid local adaptation and sex in varying habitat types in rotifers. Ecology and Evolution 3: 4253–4264.
Smith, H. A. & T. W. Snell, 2012. Rapid evolution of sex frequency and dormancy as hydroperiod adaptations. Journal of Evolutionary Biology 25: 2501–2510.
Smith, H. A. & T. W. Snell, 2014. Differential evolution of lifespan and fecundity between asexual and sexual females in a benign environment. International Review of Hydrobiology 99: 117–124.
Stelzer, C. P., 2011. The cost of sex and competition between cyclical and obligate parthenogenetic rotifers. American Naturalist 177: 43–53.
Stoks, R., L. Govaert, K. Pauwels, B. Jansen & L. De Meester, 2016. Resurrecting complexity: the interplay of plasticity and rapid evolution in the multiple trait response to strong changes in predation pressure in the water flea Daphnia magna. Ecology Letters 19: 180–190.
Tarazona, E., E. M. García-Roger & M. J. Carmona, 2017. Experimental evolution of bet hedging in rotifer diapause traits as a response to environmental unpredictability. Oikos 126: 1162–1172.
Walczyńska, A., L. Franch-Gras & M. Serra, 2017. Empirical evidence for fast temperature-dependent body size evolution in rotifers. Hydrobiologia 796: 191–200.
Wan, Q., Z.-Y. Huang, K. Zhang & Y.-L. Xi, 2022. Evolution of a primary consumer in response to low and high food availability shapes life history traits and population demography. Hydrobiologia 849: 929–943.
Wang, M., Y.-L. Xi, B. Zhou & Y. Zhang, 2014. Adaptation of Brachionus calyciflorus (Rotifera) from Lake Jinghu in summer to water temperature: population growth experiment study. Acta Hydrobiologie Sinica 38: 1017–1023.
Wen, X.-L., Y.-L. Xi, F.-P. Qian, G. Zhang & X.-L. Xiang, 2011. Comparative analysis of rotifer community structure in five subtropical shallow lakes in east China: role of physical and chemical conditions. Hydrobiologia 661: 303–316.
Wen, X.-L., Y.-L. Xi, G. Zhang, Y.-H. Xue & X.-L. Xiang, 2016. Coexistence of cryptic Brachionus calyciflorus (Rotifera) species: roles of environmental variables. Journal of Plankton Research 38: 478–489.
Xi, Y.-L., K.-Q. Huang, L. Pan, H. Zhu, Y.-L. Ge, X.-L. Wen & X.-L. Xiang, 2019. Rapid adaptation of Brachionus angularis (Rotifera) to invasion by Brachionus calyciflorus. Hydrobiologia 844: 31–42.
Xiang, X.-L., Y.-L. Xi, X.-L. Wen, G. Zhang, J.-X. Wang & K. Hu, 2011. Genetic differentiation and phylogeographical structure of the Brachionus calyciflorus complex in eastern China. Molecular Ecology 20: 3027–3044.
Xie, P., Y.-L. Xi, X.-L. Wen, J. Zhou, Y. Li, X.-X. Niu, A.-M. Wang & J.-X. Wang, 2015. Responses of the spatio-temporal dynamics of rotifer community structure to the concentrations of N and P, and the effect of top-down in two lakes. Acta Ecologica Sinica 35: 4763–4776.
Zhang, Y., A. Zhou, Y.-L. Xi, Q. Sun, L.-F. Ning, P. Xie, X.-L. Wen & X.-L. Xiang, 2018. Temporal patterns and processes of genetic differentiation of the Brachionus calyciflorus (Rotifera) complex in a subtropical shallow lake. Hydrobiologia 807: 313–331.
Zhang, C., E. Goitom, K. Brans, L. De Meester & R. Stoks, 2022. Scared to evolve? Non-consumptive effects drive rapid adaptive evolution in a natural prey population. Proceedings of Royal Society B 289: 20220188.
Zhu, H., Z.-Y. Huang, S. Jiang, L. Pan & Y.-L. Xi, 2022. Rapid adaptation of Brachionus dorcas (Rotifera) to tetracycline antibiotic stress. Aquatic Toxicology 245: 106126.
Funding
This work was supported by the Natural Science Foundation of China (Grant nos. 31971562, 31470015) and University Synergy Innovation Program of Anhui Province (Grant no. GXXT-2020-075).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by LP and HZ. The first draft of the manuscript was written by LP and Y-LX, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that is relevant to the content of this article.
Ethical approval
The manuscript has not been submitted to more than one journal for simultaneous consideration, or published elsewhere in any form or language (partially or in full). The submitted work is original and presents all results of a single study clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. All authors adhered to discipline-specific rules for acquiring and processing data. No data, text, or theories by others are presented as if they were the authors’ own. Proper acknowledgements to other works have been given. No material is verbatim copied.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Maria Špoljar, Diego Fontaneto, Elizabeth J. Walsh & Natalia Kuczyńska-Kippen / Diverse Rotifers in Diverse Ecosystems
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, L., Zhu, H., Wang, Y. et al. Adaptation of a prey population to increasing predation risks. Hydrobiologia 851, 3097–3107 (2024). https://doi.org/10.1007/s10750-023-05369-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05369-2