Abstract
Dispersal success is crucial for the survival of species in metacommunities. Zooplankton species engage in dispersal through time (i.e., egg bank) and space (i.e., vectors) by means of resting eggs. However, dispersal to patches does not equate to successful colonization, as there is a clear distinction between dispersal rates and successful colonization. We performed a field mesocosm experiment assessing dispersal and colonization success of zooplankton from resting eggs or transport via directional wind/airborne and biotic vectors in the vicinity of three ponds. By using active vs. sterile pond sediments and mesh-covered vs. open mesocosms, we disentangled the two mechanisms of dispersal, i.e., from the egg bank vs. space. We found that for both rotifers and cladocerans, sediment type, mesh cover and duration of the experiment influenced species richness and species composition. The relative contribution of resting stages to dispersal and colonization success was substantial for both rotifers and cladocerans. However, wind/airborne dispersal was relatively weak for cladocerans when compared to rotifers, whereas biotic vectors contributed to dispersal success especially for cladocerans. Our study demonstrates that dispersal and colonization success of zooplankton species strongly depends on the dispersal mode and that different dispersal vectors can generate distinct community composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal plays an integral role in structuring biological communities, especially for isolated habitats (Maguire, 1963; Schlägel et al., 2020). Sets of communities linked by the movement of multiple interacting species are termed metacommunities (Wilson, 1992; Leibold et al., 2004). Dispersal within such a metacommunity influences community composition and dynamics, the gene flow among populations and allows for colonization of new habitats (Jenkins & Buikema, 1998; Bohonak & Jenkins, 2003; Schlägel et al., 2020). In a pond metacommunity, many passively dispersing species like zooplankton rely on dispersal vectors (abiotic and biotic) to colonize new patches. These vectors support the connectivity between individual communities of aquatic organisms among ponds (Allen, 2007; Vanschoenwinkel et al., 2008a, b). Important abiotic vectors for zooplankton are water (i.e., hydrochory, Vanschoenwinkel et al, 2008c; Liu et al., 2013) and wind (i.e., anemochory, Cáceres & Soluk, 2002; Vanschoenwinkel et al., 2007) which may act on a large spatial scale. Biotic vectors (i.e., mobile linkers or zoochory, Lundberg & Moberg, 2003; Jeltsch et al., 2013) include birds (Figuerola & Green, 2002; Green & Figuerola, 2005), amphibians (Bohonak & Whiteman, 1999), and mammals (Allen, 2007; Vanschoenwinkel et al., 2008b, 2011) which may not only transport zooplankton but also floating macrophytes with their fur and feathers. The presence of floating macrophytes in new empty habitats gives an indication of the translocation of various other species by biotic vectors (Colangeli, 2018). All these dispersal vectors can contribute to a spatial homogenization of neighboring communities and allow for the colonization of new patches, however, their relevance for dispersal might be species- and vector-specific.
In addition to the dispersal in space (De Meester et al., 2005; Vanschoenwinkel et al., 2008a), zooplankton species engage in dispersal in time, when resting eggs are deposited into the sediment until favorable conditions resume and hatching starts (Brendonck & De Meester, 2003; Bilton et al., 2001; Brendonck et al., 2017). This is comparable to a seed bank in plants. Resting stages can remain viable over decades and even centuries (Hairston et al., 1995; Frisch et al., 2014). The strategy to produce resting stages is of particular relevance in temporary ponds, where they serve as a means to survive dry phases and play a significant role in fast recolonization after rewetting (Incagnone et al., 2015, Fryer, 1996; Brendonck et al., 2017). Fast recolonization from the in situ egg bank may hinder the establishment of spatially dispersing animals via priority effects (De Meester et al., 2002; Lopes et al., 2016). The Monopolization Hypothesis proposed by De Meester et al. (2002, 2016) suggests that early colonists develop large, rapidly adapting populations which impede further immigration. Thus, dispersal to patches does not equate to successful colonization, as there is a clear distinction between dispersal rates and successful dispersal for zooplankton species (Louette & De Meester, 2004).
To date, few studies have attempted to quantify the relative role of wind dispersal, biotic vectors (i.e., mobile linkers), and hatching from sediment on the colonization of zooplankton communities. Lopes et al. (2016) found that rotifers and crustaceans can colonize new patches (i.e., mesocosms) from the active egg bank and also via wind dispersal. In their study, species richness in crustaceans was lowest when only wind dispersal was allowed, and highest, when wind dispersal together with hatching from an egg bank was possible. In another dispersal study, where habitat accessibility was varied with cover meshes of different sizes to allow for size-specific biotic vectors to facilitate dispersal, no significant effect was found between mesh size (i.e., size-specific biotic vectors) and colonization (Cáceres & Soluk, 2002).
Here, we studied the role of zooplankton recruitment from resting stages stored in the sediment and biotic vectors on the colonization of new habitat patches (i.e., mesocosms) in an agricultural landscape comprising a pond metacommunity system. We had evidence from a previous study in the same study area that vertebrate biotic vectors contribute to zooplankton dispersal (Colangeli, 2018). Therefore, we investigated the colonization success of empty habitat patches and patches with egg bank, allowing for spatial dispersal via wind, biotic vectors, and dispersal in time from the resting eggs stored in the sediment in the vicinity of three ponds. This was done using 48 mesocosms in a full factorial design with fertile (i.e., viable resting stages) and sterile (i.e., dead resting stages) sediment and with and without a mesh, that excluded—if present—vertebrate biotic vectors out. We hypothesize that:
-
(1)
Fertile sediment acts as an active egg bank for rapid colonization, leading to a diverse zooplankton community.
-
(2)
Open mesocosms with sterile sediment will attract vertebrate biotic vectors dispersing zooplankton by zoochory, which requires more time than hatching from an egg bank and results in lower species richness.
-
(3)
The highest numbers of species will be found in mesocosms with fertile sediment (i.e., viable egg bank) and zoochory.
Materials and methods
Study area
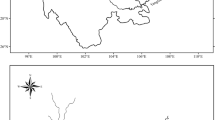
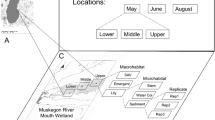
The mesocosm experiments were performed from May to August 2019 in the Agricultural Quillow catchment of the Uckermark region in North-Eastern Germany (53.2170° N, 13.8405° E) (Fig. 1). The landscape is a moraine lowland where ponds are an important part of freshwater resources. The ponds are of glacial origin dating back to the Neolithic period where ice cap fragments compressed the soil and left depressions behind (Lischeid & Kalettka, 2012). The surrounding arable land has a long history of intensive agriculture, and the ponds are characterized by high nutrient input of anthropogenic origin (Serrano et al., 2017). Sampling site selection aimed to allow for dispersal (wind, biotic, and sediment). We chose three endorheic fishless freshwater ponds with a history of a stable hydroperiod, which however dried out or shrank to muddy puddles due to long-lasting drought during the experimental period: Pond 807 (size: 1047 m2) dried out in August, pond 1598 (2526 m2) dried out in May, and pond 2484 (10603 m2) dried out in July (Kiemel et al., 2022). The ponds are in a geographic range of ~ 14 km situated within a triticale field and represent a subset of broader studies in the same region (Colangeli, 2018; Onandia et al., 2021; Kiemel et al., 2022). During the experimental period, all three ponds were sampled monthly and the zooplankton composition was analyzed using DNA metabarcoding (Kiemel et al., 2022).
Experimental design
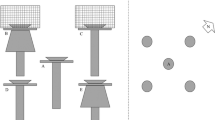
We studied three relevant factors for zooplankton colonization of mesocosms, i.e., sediment (with active egg bank vs. sterile), wind, and biotic vectors (animals). We manipulated the exclusion of biotic vectors (B) and the exclusion of the egg bank (E) in a 2 × 2 factorial design. Wind dispersal was possible in all cases. The four experimental groups were; (a) exclusion of the egg bank by the provision of sterile sediment, and exclusion of biotic vectors by covering with a mesh (E−B−), (b) fertile egg bank: sediment with active egg bank and exclusion of biotic vectors by mesh cover (E + B−), (c) biotic vectors: sterile sediment in open mesocosm (E−B +), and (d) egg bank and biotic vectors: fertile sediment in open mesocosm (E + B +). Within each set of four mesocosms, the treatments were placed randomly, and each set was placed in the four wind cardinal points (North, South, East, West) of the three ponds: 16 for each pond, resulting in a total number of 48 mesocosms (Fig. 2).
We performed the experiment with white plastic mesocosms of 30 L volume (Ø 50 cm). Prior to the experiments, mesocosms were thoroughly washed and scrubbed to eliminate any organisms or resting stages. We then added a 3 cm layer of sediment collected from the selected three ponds to their corresponding mesocosms to serve as resting egg banks similar to Waterkeyn et al. (2010) and Lopes et al. (2016). Two cores of the first 5 cm of the active egg bank (sediment) (Brendonck & De Meester, 2003; Kiemel et al., 2022) were collected from different parts of the ponds within a 0.5 m2 rectangular quadrant using a round Gardena® bulb planter with Ø 8 cm (amounting for 500 cm3 sediment per 0.5 m2 site with 70 sites per pond). The collected sediment from each pond was carefully mixed and treated separately. For the experimental groups with “active” resting egg bank (E +), sediments were dried in an oven (BINDER FD 115-E2) at 30 °C for 72 h. For treatments with sterile sediment (E−), the sediment was frozen at -18 °C for 96 h, thawed and refrozen (Emmerson et al., 2001; Lopes et al., 2016) and then autoclaved and subsequently dried at 30 °C for 72 h with the purpose of killing all organisms including resting stages. Mesocosms were filled with tap water (Jenkins & Buikema, 1998) to give a consistent and high nutrient base for all experimental groups, allowing for rapid microalgal growth as food. For experimental groups B−, a 4 mm mesh cover was tightly placed on top of the mesocosms to prevent large vertebrates such as raccoons, deer, and wild boars from interacting with the mesocosms. Wind dispersal was not prevented in any of the treatments, so that it can be assumed that organisms colonizing treatment E−B− were dispersed by wind. The four sets of mesocosms were placed ca. 2 m from the edge of each pond (Fig. 2). The average wind direction was recorded using an anemometer (Vantage Pro2™, Davis) throughout the experiment and was predominantly toward South (169° ± 57 SD) with an average wind speed of 1.7 m s−1 ± 1.3 SD. The height of the mesocosms from the ground was 42 cm as this allowed mainly large mammals (deer, raccoons, and wild boars) and birds to access open mesocosms since these species were our target biotic "dispersers". We set up camera traps (Reconyx Hyperfire HC500™) near the ponds to detect potential mesocosm visits from biotic vectors.
Community samples
The zooplankton community was sampled every 15 days on six sampling campaigns (days 15, 30, 45, 60, 75, and 90). A time interval of 15 days is in the range when the first airborne dispersal can be detected (Colangeli, 2018). Before sampling, we thoroughly mixed the water, collected a 3 L sample with a measuring cylinder and filtered it through a 30 μm mesh funnel placed above the opening. The filtrate re-entered the mesocosms, hence no water was lost with this procedure. We used different funnels for each mesocosm, and thoroughly rinsed them in deionized water to prevent contamination. For each sample, we transferred the concentrated volume of 20 mL into a 50 mL vial and added 30 mL of 95% EtOH for fixation (Cáceres & Soluk, 2002; Black & Dodson, 2003). Zooplankton species were morphologically identified to the lowest possible taxonomic unit (Voigt, & Koste, 1978; Bledzki & Rybak, 2016) using an epifluorescent microscope (Zeiss Axioskop 2, Germany) and for accurate identification, we stained the trophi of rotifer specimens with Calcofluor white (Fig. S1, see Supplementary Information). The same volume of triplicate aliquots of zooplankton samples were analyzed for all samples using a Sedgewick-Rafter cell. As abiotic factors, potentially influencing colonization we measured temperature (Portamess® 911, Knick) and pH (Portamess® 911, Knick) for each mesocosm. Macrophytes found in mesocosms, either germinating from the sediment or transported into sterile mesocosms, were photographed during the course of the experiment, collected after the duration of the experiment (90 days), stored in 4 °C and identified using taxonomic keys from Jäger (2017).
Furthermore, Kiemel et al. (2022) sampled 24 ponds in the study area (including our three selected ponds) during the same experimental period and analyzed the community composition using a two-fragment DNA meta-barcoding approach. This data allows for comparisons of species identified in ponds with those identified in the mesocosms. However, since the ponds of the present study partially dried out, the field data did not cover the full experimental period. In addition, species pool data from microscopical analysis from 2016 of 20 ponds (Colangeli, 2018) were used.
Statistical analyses
Statistical analysis was performed in R 4.1.2 (R Core Team, 2021) and Excel (2016). Separate analyses were performed on data for our two target taxa (rotifers and cladocerans). We used GLMM models (Gelman & Hill, 2006; Zuur et al., 2009) [function “glmer,” package lme4 (Bates et al., 2015)] to investigate the effect of background parameters (time (days), mesh cover (B+, B−), sediment type (E+, E−), and mesocosm directional location) on species richness of rotifers and cladocerans. Since there were multiple and repeated observations from each pond, we used linear mixed-effect models with nested parameters of pond ID (random effect). We used the presence of floating macrophytes in sterile soils as an indication of realized biotic dispersal (i.e., mobile linkers). The positions or locations of the mesocosms in the cardinal points (N, S, E, W) were used as proxy to measure directional wind dispersal as it is assumed that mesocosms in the South of each pond will intercept propagules transported by wind blowing from the North. Identification of the best model was conducted based on the Akaike information criterion (AIC), using the dredge function in the R package MuMIn (Barton, 2016). We confirmed the normality of the model residuals via QQ-plots (Fig. S3 and Fig. S4, see Supplementary Information).
To analyze the rate of colonization and species turnover, we calculated the cumulative species richness over time. To compare the community composition we used the PERMANOVA test [adonis functions available in the vegan package, (Oksanen et al., 2022)] with 9999 permutations (Blanchet et al., 2008, 2009) for each sampling date separately with treatment mesh cover (B+, B−) and sediment type (E+, E−) as fixed effects and pond ID as a random effect. The analyses were based on abundances using Bray–Curtis distances and a non-metric multidimensional scaling (NMDS) approach was applied to visualize differences in species composition of invertebrate taxa with significant parameters of community composition. We used the same parameters stated above in the mixed model. Additionally, we used post hoc multilevel pairwise tests [pairwise.adonis function] with Bonferroni correction to assess the significance among the group treatments and ponds (see Supplementary Information). Stress plots of NMDS analyses are shown in Fig. S5 and Fig. S6 (see Supplementary Information).
Results
Water temperatures in mesocosms fluctuated monthly from a low of 16.5 °C to a high of 29 °C but did not differ among treatments with the maximum difference among mesocosms over the entire period of the experiment being 2.5 °C. The pH of mesocosms also varied with time, ranging from 7.35 and 10.35. In the beginning of the experiment, mesocosms with active egg bank (E+) had a slightly lower pH (mean 8.3) as compared to the ones with sterile sediment (E−) (mean = 8.8). Toward the end of the experiment, these differences leveled off. Camera traps captured mammals (e.g., raccoon, deer, and wild boar) and birds (Fig. S2) drinking from open mesocosms. We were not able to fully record all mesocosms during the entire experimental period, as some cameras were damaged or the lens was obscured by plants, thus making it impossible for us to quantitatively assess when and by whom which mesocosms were visited.
Microalgae colonized all mesocosms quickly (i.e., visible by the greenish coloring of the water) providing food for zooplankton. Over the 90-day period, we identified in total 14 aquatic macrophyte species (Supplementary Information, Table S1) in the mesocosms. The first appearance of macrophyte species was during the 2nd sampling campaign (i.e., 30 days) in experimental groups with active egg bank (E+) comprising substrate-bound and floating species. We found exclusively floating plants (Lemna minor Linnaeus, 1753, Lemna gibba Linnaeus, 1753 and Lemna sp.) in experimental group E−B+ from 5th sampling period (75 days), while no aquatic macrophyte species were found in mesocosms in the experimental group E−B−. After the establishment of the floating macrophytes, they covered more than 50% of the water surface within 14 days.
We focused on the two major metazoan zooplankton groups; rotifers and cladocerans as they were our target group. There was little colonization observed in enclosures with egg banks (E +) during the initial 15 days of the experiment. We identified 83 rotifer species and 18 cladocerans species (Fig. 3) in the mesocosms during the experimental period. The most common rotifers identified were Cephalodella catellina Müller, 1786 (found in 45 mesocosms), Trichocerca weberi Jennings, 1903 (found in 40 mesocosms), Lecane closterocerca Schmarda, 1859 (found in 38 mesocosms) and Bdelloid sp. (found in 35 mesocosms). Of the 83 rotifer species, we recorded 75 species in experimental group E+B+, 71 species in E+B−, 39 species in E−B+, and 43 species in E−B− (Fig. 4). Experimental groups with active egg bank (E+) contained 29 unique rotifer species (see Supplementary Information, Table S2) compared to sterile sediment groups (E−) and predatory rotifers (such as Asplanchna brightwellii Gosse, 1850, Asplanchna girodi de Guerne, 1888, Asplanchna pridonta Gosse, 1850, and Asplanchna sieboldi Leydig, 1854) were solely found in mesocosms with viable egg bank.
Relative abundances of zooplankton species a) for rotifers b) for cladocerans from the mesocosms. Treatments: E−B− = Sterile sediment and exclude biotic vectors, E−B+ = Sterile sediment and allow biotic vectors, E + B− = Egg bank and exclude biotic vectors, E + B + = Egg bank and allow biotic vectors
For cladoceran species, the most common species recorded were Alona sp. (found in 34 mesocosms), Ceriodaphnia reticulata Jurine, 1820 (found in 32 mesocosms), Ceriodaphnia dubia Richard, 1894 (found in 32 mesocosms), and Ceriodaphnia quadrangula Müller, 1785 (found in 29 mesocosms). Out of 18 cladoceran species, we detected all 18 species in both E−B+ and E + B− experimental groups, 15 species in E−B+, and 10 species in E−B−. Experimental groups with egg bank (E +) recorded three unique cladocerans species compared to sterile sediment groups (E−) (see Supplementary Information, Table S2). With the sterile sediment groups E−, we observed five more cladocerans species in E−B + (open mesocosms) when compared to mesh-covered mesocosms (E−B−). Species abundance increased with the sampling period for most treatments. The experimental group with the highest mean abundance of 701 Ind/L for rotifers and 62 for Ind/L for cladocerans over the experimental period was the wind only dispersal (E−B−) (Fig. 5).
Zooplankton abundance (mean ± SD) over time in the mesocosms. a) For rotifers, b) for cladocerans. Treatments: E−B− = Sterile sediment and exclude biotic vectors, E−B+ = Sterile sediment and allow biotic vectors, E + B− = Egg bank and exclude biotic vectors, E + B + = Egg bank and allow biotic vectors. Unit: Individuals per liter (Ind/L)
Comparing the total zooplankton community in all 48 mesocosms with the regional species pool from a survey from 2016 (Colangeli, 2018), 61% of the mesocosms species were also found in at least one out of 20 sampled ponds. Thus, 39% of the species from the 2019 mesocosms were not detected in the field three years earlier by monthly sampling and microscopic analysis. The pond-specific species overlap between the simultaneously taken pond samples (DNA meta-barcoding) and the mesocosms was even lower (Kiemel et al., 2022) (Fig. 6).
Species richness
Overall, rotifers and cladocerans showed an opposing trend over time. Rotifer species richness declined after a peak at day 30 and cladoceran richness increased toward the end (day 90; Fig. 7). Sediment type (i.e., egg bank (E +) or sterile (E–)) had a significant effect on species richness of rotifers (Table 1). There was a higher species richness for experimental groups with egg bank (E + B + and E + B−) in the beginning for both rotifers and cladocerans, however by the end of the experimental period, species richness was similar for all groups, except for a low cladoceran species richness in E−B− (Fig. 7). Macrophytes found in mesocosms with egg bank (E + B + and E + B−) consisted of both substrate-bound species (such as Alisma plantago-aquatica Linnaeus, 1753 and Sparganium erectum Linnaeus, 1753) and floating species (e.g., L. minor and L. gibba), while macrophytes in the sterile open group (E−B+) were exclusively floating ones. The zooplankton species richness was higher when macrophytes were present (Fig. 7). This difference is most prominent with cladocerans, as species richness increased with macrophyte appearance in sterile open mesocosms (E−B+) in comparison to sterile mesh-covered mesocosms (Fig. 7). We observed a significant effect of mesh cover (B + vs. B−) on species richness only for cladocerans, with higher species richness in open mesocosms (no mesh cover, allow biotic vectors) (Table 1). Thus, active sediment served as an efficient source for rotifers and cladocerans, whereas the latter also benefitted from biotic vectors. We did not find an effect of the directional position (location) of the mesocosms on species richness, neither for rotifers nor for cladocerans. The mean cumulative species richness curves (Fig. 8) reveal a high species colonization rate for rotifers from E + mesocosms, which had almost reached saturation after 45 days. Thus, the decline in species richness in these treatments is the result of species extinction without colonization of new species indicating no species turnover. The cumulative species richness in the E− treatments increased slightly until the end of the experiment despite relatively constant species richness in the individual mesocosms from day 60 onwards. For cladocerans, the temporal pattern of species richness and cumulative species richness is very similar. The cumulative species richness of the E- treatments is constantly increasing until day 90, whereas the richness increased only moderately in the E + treatments (Fig. 8).
Species richness (mean ± SD) for rotifers (a) and cladocerans (b) in the mesocosms. When macrophytes were observed in the sterile sediment treatments, species richness was separated in (c) rotifers and (d) cladocerans according to the presence/absence of macrophytes. Treatments: E−B− = Sterile sediment and exclude biotic vectors, E−B + = Sterile sediment and allow biotic vectors, E + B− = Egg bank and exclude biotic vectors, E + B + = Egg bank and allow biotic vectors
Cumulative species richness (mean ± SD) for the four treatments over time. a) for rotifers, b) for cladocerans. Treatments: E−B− = Sterile sediment and exclude biotic vectors, E−B + = Sterile sediment and allow biotic vectors, E + B− = Egg bank and exclude biotic vectors, E + B + = Egg bank and allow biotic vectors
Species community composition
Rotifers and cladocerans showed different patterns of species composition during the experimental time, as revealed by a PERMANOVA (Table 2). For rotifers, mesh cover and sediment type had a significant effect on species community composition depending on the sampling day (Table 2). There was a consistent effect of sediment type on species composition from day 15 to day 90. Mesh cover had a significant effect on species composition only for samples taken at day 75. We found a convergence of treatment groups by day 90 (Fig. 9).
NMDS ordination plots based on species community composition of rotifers for each of the sampling period with centroid mean and 50% CI ellipse. Each dot represents a mesocosm during a sampling period. Treatments: E−B− = Sterile sediment and exclude biotic vectors, E−B + = Sterile sediment and allow biotic vectors, E + B- = Egg bank and exclude biotic vectors, E+B+ = Egg bank and allow biotic vectors. Data from the first sampling date (i.e., after 15 days) were not analyzed because most of the enclosures were not colonized at that time. Note: different scales for axes
For cladocerans, mesh cover and sediment type significantly influenced community composition depending on the sampling day (Table 2). There was a consistent effect of sediment type on species composition from day 45 to day 90. Mesh cover had a significant effect on species composition for samples taken on day 90. The mesocosms that allowed only wind dispersal (E−B−) deviated from the others until day 60, and convergence of all treatment groups was observed at day 90 (Fig. 10).
NMDS ordination plots based on species community composition of cladocerans for each of the sampling period with centroid mean and 50% CI ellipse. Each dot represents a mesocosm during a sampling period. Treatments: E−B− = Sterile sediment and exclude biotic vectors, E−B+ = Sterile sediment and allow biotic vectors, E+B— = Egg bank and exclude biotic vectors, E+B+ = Egg bank and allow biotic vectors. Data from the first and second sampling dates (i.e., after 15 and 30 days) were not analyzed because most of the enclosures were not colonized at that time. Note: different scales for axes
Discussion
In line with our hypotheses, we found fertile sediment (i.e., egg bank) was a driving force of species community structure and composition for both rotifers and cladocerans. Biotic vectors visited open mesocosms and dispersed species, with this mode of dispersal being significantly important for cladocerans.
Species richness and colonization
In our study, zooplankton colonized the mesocosms over the course of the experiment via egg bank, airborne dispersal, and biotic vectors, but we found taxon-specific variation in the amount of time needed for arrival. The first colonizing species found on day 15 were rotifers from the egg bank, rapidly increasing until day 30 and declining continuously afterward. For cladocerans, only a few colonizing species were observed after 30 days, however, species numbers were slowly but continuously increasing until day 90. This disparity in colonization dynamics between rotifers and cladocerans suggests that cladocerans have lower colonization rates and lower growth rates leading to an overall delayed community establishment. In general, rotifers have shorter generation times and can reach higher population densities as compared to cladocerans (Finlay, 2002; Cohen & Shurin, 2003). In accordance with our findings, Lopes et al. (2016) and Cáceres & Soluk (2002) reported rotifers as the first colonizers and the rapid growth of the rotifer community, followed by the development of the cladoceran community.
We found a high abundance of rotifers in wind only treatments (E− B−) which can be attributed to priority effects: First colonizers in this treatment had no competitors or predators and increased rapidly in population size (De Meester et al., 2002). The dominance of very few species could have been facilitated by species-specific/taxon-specific differences in dispersal capacity/limitation (Cáceres & Soluk, 2002).
Dormant resting eggs hatched and contributed substantially to the high rotifer species richness observed on day 30 in the mesocosms with egg bank, however, there was a subsequent decrease over time. In comparison, mesocosms with sterile sediment began with low species richness and increased with time, until richness in both experimental groups converged at the end of the experiment. The final total number of individuals and species in the mesocosms is likely driven also by local factors such as competition and/or predation (Louette & De Meester, 2004). In well-established communities, local biotic interactions like competition, predation and parasitism drive the community structure, rather than dispersal (Shurin, 2000). Competition might be high in mesocosms with an egg bank, as species must compete with simultaneously hatching individuals. Competition acts mainly through food availability; however, no data are available for our system to estimate a possible food limitation. Since rotifers differ considerably in their preferred food, bulk measurements of e.g., chlorophyll would provide only weak evidence for potential food limitation. In general, large daphnids (e.g., Daphina magna Straus, 1820), as were initially observed in mesocosms with egg bank only, can suppress rotifer populations, as they replace rotifers during the seasonal succession in field and experimental studies (Gilbert, 1988). Predation might have been another factor driving rotifer species richness (Sih et al., 1985). In our study, predatory rotifers such as Asplanchna spp. were exclusively observed in mesocosms with a viable egg bank. However, our data do not allow for a quantification of either competition or predation. Jenkins & Buikema (1998) and Cáceres & Soluk (2002) observed an increase in species richness initially until there was a plateau after some months in newly created ponds. Lopes et al. (2016) also reported a convergence of rotifer species richness among different experimental groups after 53 days. In our experiment, the decrease in species richness in E + treatments could be attributed exclusively to species extinctions, after the cumulative species richness had already reached its saturation. The slight increase of cumulative species richness in E− treatments along with constant species numbers in the individual mesocosms point to ongoing colonization and species turnover. For cladocerans, there was an increase in species richness over time for mesocosms with viable egg bank compared to sterile ones, however, the appearance of macrophytes coincided with the increase in cladoceran species richness. Since these macrophytes were exclusively floating species such as Lemna sp. that are likely dispersed by biotic vectors, we assume that cladocerans were co-dispersed with these macrophytes (Allen, 2007; Vanschoenwinkel et al., 2011; Colangeli, 2018). Biotic vectors (i.e., mobile linkers) are one effective way of passive dispersal for zooplankton species as has been demonstrated in other studies as well (Bohonak & Whiteman, 1999; Figuerola & Green, 2002; Frisch & Green, 2007; Vanschoenwinkel et al., 2008b). We observed increased rotifer and cladoceran species richness in these open mesocosms. The open mesocosms were frequently visited by various mammals such as raccoons, deer, foxes, weasels, and wild boars, as well as by songbirds and storks, potentially transporting macrophytes along with zooplankton species (Fig. S2, see Supplementary Information). Some cladoceran species (D. magna, Daphnia longispina Müller, 1776, Daphnia pulex Leydig, 1860) were initially exclusively observed in mesocosms with egg bank, but allowing for zoochory, they were later also observed in open sterile mesocosms. In line with our findings, Allen (2007) reported successful dispersal of zooplankton in open mesocosms, where there were frequent visits by animal vectors (such as raccoons, opossum, and deer). Contrarily, Cáceres & Soluk (2002) did not find a clear difference on colonization rates between mesh-covered and open mesocosms, after frequent visits from biotic vectors. Thus, we suggest that the regional environment determines the relative role of biotic vectors for zooplankton dispersal.
The species number of cladocerans were low in the wind dispersal only treatment. Colonization by cladocerans in this treatment occurred first after 60 days, indicating substantial dispersal limitation. There are some explanations as to why cladocerans are not readily dispersed by wind: their propagules are relatively large and have specific traits such as sticky envelopes or hooks for firm attachment to vegetation (Fryer, 1996; Brendonck & De Meester, 2003), which may reduce their airborne dispersal (Fryer, 1996). Another limitation is the low abundance of cladoceran propagules relative to rotifers that can compromise the detection of airborne dispersal. Studies have reported that the density of rotifer propagules in pond sediments outweighs those of cladocerans (Santangelo et al., 2015). This has been attributed to the longer generation times and smaller population sizes of cladocerans, making propagules less available for propagation (Cohen & Shurin, 2003). Thus, even though some cladocerans (e.g., Daphnia pulex Leydig, 1860 and Simocephalus spp.) have relatively low lift-off wind velocity (Pinceel et al., 2016), their low propagule abundance and availability can limit dispersal (Vanschoenwinkel et al., 2008a). The overall slow and stochastic dispersal of cladocerans by wind and animals is also reflected in the cumulative species richness curves. They show continuous colonization by new species even when species richness no longer increased, which suggests that the new colonizers have also replaced some earlier ones.
These findings suggest that cladocerans rely mainly on biotic vectors for successful dispersal, whereas rotifers colonized sterile mesh-covered mesocosm (wind/air-borne only) after 30 days. Due to their relatively small size, rotifer propagules can be easily transported by wind (De Bie et al., 2012; Lopes et al., 2016), which explains their early colonization in all mesocosms.
Species composition and community structure
In our study, the community structure varied with time as colonization success of species differed. Priority species such as rotifers are the first to colonize new patches and with time the slow colonizers such as cladocerans follow and have the potential to replace them (Gilbert, 1988). Thus, local processes such as succession, predation, and competition likely played a role.
We found differences in species composition among some group treatments for both rotifers and cladocerans. The different pathways of dispersal seemed to influence the colonization success of zooplankton species into new patches and generated distinct communities (Cáceres & Soluk, 2002; Cohen & Shurin, 2003). Recolonization of patches by resting stages is very effective in establishing populations (Brendonck & De Meester, 2003; Brendonck et al., 2017) as compared to most spatial dispersal ways. Abiotic vectors such as wind play a role in the overland dispersal of species on small scales (e.g., Cáceres & Soluk, 2002; Sciullo & Kolasa, 2012), however it results in lower rates of successful colonization because individuals are deposited randomly across the landscape instead of directed to suitable habitats (Cohen & Shurin, 2003). Thus, wind dispersal serves as a process of maintaining species diversity (Jenkins & Buikema, 1998), while dispersal in time serves as a process of maintaining established populations/communities (De Stasio, 1989).
Although we observed differences in community composition among ponds, we cannot attribute any difference to isolation by distance. We have only three ponds located within a small range of 14 km, with the geographically farthest two ponds having a similar community structure. The temporal scale of our study, 90 days, does neither allow for conclusions about dispersal limitation on a regional scale nor at a time scale of decades or even longer. However, the colonization of the mesocosms by so many species suggests that, on a longer time scale, dispersal limitation is not an important driving factor for total species richness in our system.
Comparison between ponds and mesocosms
Overall, we found more species in the mesocosms than in the adjacent ponds (Fig. 6). This can be attributed to several reasons. Firstly, some species might have entered the mesocosms that did not originate from the pond next to the mesocosms, for example, they were dispersed over larger distances. Secondly, since the ponds partially dried out, the pond data cover only a limited part of the experimental period. In former years, the ponds were classified as permanent so it can be expected that the egg bank comprised species from a whole season, whereas the pond species number was lower because of the early dryout. Thirdly, the environmental conditions in the mesocosms and in the ponds differed so the species composition might be different because of environmental filtering. Lastly, the two methods, DNA meta-barcoding and microscopic analyses might not yield 100% congruence.
Implication for metacommunity structure
Our study within an agricultural landscape indicated that the dispersal of zooplankton was mediated via resting stages, wind, and animals, enabling the colonization of new habitats. Not all zooplankton species were readily dispersed, with the difference in colonization rates due to either an intrinsic difference in dispersal capacity or to a lower establishment success (Cohen & Shurin, 2003; Louette & De Meester, 2004). Although we observed high dispersal of species, our results show that the first 60 days of community buildup were strongly influenced by dispersal limitation, especially in experimental setups without egg banks. This is evident from the slow increase in species richness of cladocerans. Contrarily, we observed the opposite for rotifers in the experimental setups with egg bank with a consistent decline in species richness indicating local processes such as competition and predation (Louette & De Meester, 2004). The outcome suggests that both dispersal limitation on a short time scale and local processes influence community structure depending on the time, zooplankton group, and pathways of colonization.
The overall joint effects of spatial (i.e., wind and animals) dispersal and dispersal in time (i.e., resting stages) maintain connectivity (Allen, 2007; Vanschoenwinkel et al., 2008a, b) among habitats, shaping the community structure of passively dispersing zooplankton species.
Conclusion
The focus of our study was to identify the contributions of resting stages and spatial dispersal (i.e., wind and animals) to community structure of zooplankton. We found that priority effects, dispersal limitations, and local factors most likely influence the zooplankton community structure. With increasing habitat fragmentation, farming practices, and dryfall of ponds due to climate change, there is a risk of depletion of resting stages and activities of biotic vectors (i.e., mobile linkers) which can halt the recovery of many species and lead to local extinction of species.
Data availability
The original data are available on Dryad Repository https://doi.org/10.5061/dryad.h70rxwdmt, further inquiries can be directed to the corresponding author.
References
Allen, M. R., 2007. Measuring and modeling dispersal of adult zooplankton. Oecologia 153: 135–143. https://doi.org/10.1007/s00442-007-0704-4.
Bates, D., M. Mächler, B. Bolker & S. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1): 1–48. https://doi.org/10.18637/jss.v067.i01.
Bilton, D. T., A. Foggo & S. D. Rundle, 2001. Size, permanence and the proportion of predators in ponds. Archiv für Hydrobiologie. https://doi.org/10.1127/archiv-hydrobiol/151/2001/451.
Binks, J. A., S. E. Arnott & W. G. Sprules, 2005. Local factors and colonist dispersal influence crustacean zooplankton recovery from cultural acidification. Ecological Applications 15: 2025–2036. https://doi.org/10.1890/04-1726.
Black, A. R. & S. I. Dodson, 2003. Ethanol: a better preservation technique for Daphnia. Limnology and Oceanography: Methods 1: 45–50. https://doi.org/10.4319/lom.2003.1.45.
Blanchet, F. G., P. Legendre & D. Borcard, 2008. Forward selection of explanatory variables. Ecology 89: 2623–2632. https://doi.org/10.1890/07-0986.1.
Blanchet, F. G., P. Legendre & D. Borcard, 2009. Erratum to “Modelling directional spatial processes in ecological data”[Ecol. Modell. 215 (2008) 325–336]. Ecological Modelling 220: 82–83. https://doi.org/10.1016/J.Ecolmodel.2008.08.018.
Bledzki, L. A. & J. I. Rybak, 2016. Freshwater crustacean zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) key to species identification, with notes on ecology, distribution, methods and introduction to data analysis. Springer. https://doi.org/10.1007/978-3-319-29871-9.
Bohonak, A. J., & D. G. Jenkins, 2003. Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecology letters 6(8): 783–796. https://doi.org/10.1046/j.1461-0248.2003.00486.x
Bohonak, A. J. & H. H. Whiteman, 1999. Dispersal of the fairy shrimp Branchinecta coloradensis (Anostraca): effects of hydroperiod and salamanders. Limnology and Oceanography 44: 487–493. https://doi.org/10.4319/lo.1999.44.3.0487.
Brendonck, L. & L. De Meester, 2003. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491: 65–84. https://doi.org/10.1023/A:1024454905119.
Brendonck, L., T. Pinceel & R. Ortells, 2017. Dormancy and dispersal as mediators of zooplankton population and community dynamics along a hydrological disturbance gradient in inland temporary pools. Hydrobiologia 796: 201–222. https://doi.org/10.1007/s10750-016-3006-1.
Cáceres, C. E. & D. A. Soluk, 2002. Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia 131: 402–408. https://doi.org/10.1007/s00442-002-0897-5.
Cohen, G. M. & J. B. Shurin, 2003. Scale-dependence and mechanisms of dispersal in freshwater zooplankton. Oikos 103: 603–617. https://doi.org/10.1034/j.1600-0706.2003.12660.x.
Colangeli, P., 2018. From pond metacommunities to life in a droplet causes and consequences of movement in zooplankton [Thesis dissertation]. Department of Ecology and Ecosystem Modelling, Universität Potsdam.
De Bie, T., L. De Meester, L. Brendonck, K. Martens, B. Goddeeris, D. Ercken, H. Hampel, L. Denys, L. Vanhecke & K. Van der Gucht, 2012. Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecology Letters 15: 740–747. https://doi.org/10.1111/j.1461-0248.2012.01794.x.
De Meester, L., A. Gómez, B. Okamura & K. Schwenk, 2002. The Monopolization Hypothesis and the dispersal–gene flow paradox in aquatic organisms. Acta Oecologica 23: 121–135. https://doi.org/10.1016/S1146-609X(02)01145-1.
De Meester, L., S. Declerck, R. Stoks, G. Louette, F. Van De Meutter, T. De Bie, E. Michels & L. Brendonck, 2005. Ponds and pools as model systems in conservation biology, ecology and evolutionary biology. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 715–725. https://doi.org/10.1002/aqc.748.
De Meester, L., J. Vanoverbeke, L. J. Kilsdonk & M. C. Urban, 2016. Evolving perspectives on monopolization and priority effects. Trends in Ecology & Evolution 31: 136–146. https://doi.org/10.1016/j.tree.2015.12.009.
De Stasio, B. T., 1989. The seed bank of a freshwater crustacean: copepodology for the plant ecologist. Ecology 70: 1377–1389. https://doi.org/10.2307/1938197.
Emmerson, M. C., M. Solan, C. Emes, D. M. Paterson & D. Raffaelli, 2001. Consistent patterns and the idiosyncratic effects of biodiversity in marine ecosystems. Nature 411: 73–77. https://doi.org/10.1038/35075055.
Figuerola, J. & A. J. Green, 2002. Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies. Freshwater Biology 47: 483–494. https://doi.org/10.1046/j.1365-2427.2002.00829.x.
Finlay, B. J., 2002. Global dispersal of free-living microbial eukaryote species. Science 296: 1061–1063. https://doi.org/10.1126/science.1070710.
Frisch, D. & A. J. Green, 2007. Copepods come in first: rapid colonization of new temporary ponds. Fundamental and Applied Limnology 168: 289–297. https://doi.org/10.1127/1863-9135/2007/0168-0289.
Frisch, D., K. Cottenie, A. Badosa & A. J. Green, 2012. Strong spatial influence on colonization rates in a pioneer zooplankton metacommunity. PLoS ONE 7: e40205. https://doi.org/10.1371/journal.pone.0040205.
Frisch, D., P. K. Morton, P. R. Chowdhury, B. W. Culver, J. K. Colbourne, L. J. Weider & P. D. Jeyasingh, 2014. A millennial-scale chronicle of evolutionary responses to cultural eutrophication in Daphnia. Ecology Letters 17: 360–368. https://doi.org/10.1111/ele.12237.
Fryer, G., 1996. Diapause, a potent force in the evolution of freshwater crustaceans. Hydrobiologia 320: 1–14. https://doi.org/10.1007/BF00016800.
Gelman, A. & J. Hill, 2006. Data analysis using regression and multilevel/hierarchical models. Cambridge University Press. https://doi.org/10.1017/CBO9780511790942.
Gilbert, J. J., 1988. Suppression of rotifer populations by Daphnia: a review of the evidence, the mechanisms, and the effects on zooplankton community structure 1. Limnology and Oceanography 33: 1286–1303. https://doi.org/10.4319/lo.1988.33.6.1286.
Gray, D. K. & S. E. Arnott, 2011. Does dispersal limitation impact the recovery of zooplankton communities damaged by a regional stressor? Ecological Applications 21: 1241–1256. https://doi.org/10.1890/10-0364.1.
Gray, D. K. & S. E. Arnott, 2012. The role of dispersal levels, Allee effects and community resistance as zooplankton communities respond to environmental change. Journal of Applied Ecology 49: 1216–1224. https://doi.org/10.1111/j.1365-2664.2012.02203.x.
Green, A. J. & J. Figuerola, 2005. Recent advances in the study of long-distance dispersal of aquatic invertebrates via birds. Diversity and Distributions 11: 149–156. https://doi.org/10.1111/j.1366-9516.2005.00147.x.
Hairston, N. G. & C. E. Cáceres, 1996. Distribution of crustacean diapause: micro-and macroevolutionary pattern and process. Hydrobiologia 320: 27–44. https://doi.org/10.1007/BF00016802.
Hanzawa, F. M., A. J. Beattie & D. C. Culver, 1988. Directed dispersal: demographic analysis of an ant-seed mutualism. The American Naturalist 131: 1–13. https://doi.org/10.1086/284769.
Havel, J. E. & J. B. Shurin, 2004. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnology and Oceanography 49: 1229–1238. https://doi.org/10.4319/lo.2004.49.4_part_2.1229.
Horváth, Z., C. F. Vad & R. Ptacnik, 2016. Wind dispersal results in a gradient of dispersal limitation and environmental match among discrete aquatic habitats. Ecography 39: 726–732. https://doi.org/10.1111/ecog.01685.
Incagnone, G., F. Marrone, R. Barone, L. Robba & L. Naselli-Flores, 2015. How do freshwater organisms cross the “dry ocean”? A review on passive dispersal and colonization processes with a special focus on temporary ponds. Hydrobiologia 750: 103–123. https://doi.org/10.1007/s10750-014-2110-3
Jäger, E.J. 2017: Rothmaler - Exkursionsflora von Deutschland. Gefäßpflanzen: Grundband. 21. ed. Springer, 930 p.
Jeltsch, F., D. Bonte, G. Pe’er, B. Reineking, P. Leimgruber, N. Balkenhol, B. Schröder, C. M. Buchmann, T. Mueller & N. Blaum, 2013. Integrating movement ecology with biodiversity research exploring new avenues to address spatiotemporal biodiversity dynamics. Movement Ecology 1: 1–13. https://doi.org/10.1186/2051-3933-1-6.
Jenkins, D. G., & A. L. Buikema Jr., 1998. Do similar communities develop in similar sites? A test with zooplankton structure and function. Ecological Monographs 68(3): 421–443. https://doi.org/10.1890/0012-9615(1998)068[0421:DSCDIS]2.0.CO;2.
Jenkins, D.G., & M. O. Underwood, 1998. Zooplankton may not disperse readily in wind, rain, or waterfowl. In: Wurdak, E., Wallace, R., Segers, H. (eds) Rotifera VIII: A Comparative Approach. Developments in Hydrobiology, 134. Springer. https://doi.org/10.1007/978-94-011-4782-8_3
Juračka, P. J., S. A. Declerck, D. Vondrák, L. Beran, M. Černý & A. Petrusek, 2016. A naturally heterogeneous landscape can effectively slow down the dispersal of aquatic microcrustaceans. Oecologia 180: 785–796. https://doi.org/10.1007/s00442-015-3501-5.
Kiemel, K., G. Weithoff & R. Tiedemann, 2022. DNA metabarcoding reveals impact of local recruitment, dispersal, and hydroperiod on assembly of a zooplankton metacommunity. Molecular Ecology. https://doi.org/10.1111/mec.16627.
Kulkarni, M. R., S. M. Padhye, R. B. Rathod, Y. S. Shinde & K. Pai, 2019. Hydroperiod and species-sorting influence metacommunity composition of crustaceans in temporary rock pools in India. Inland Waters 9: 320–333. https://doi.org/10.1080/20442041.2018.1548868.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law & D. Tilman, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x.
Levin, S. A., D. Cohen & A. Hastings, 1984. Dispersal strategies in patchy environments. Theoretical Population Biology 26: 165–191. https://doi.org/10.1016/0040-5809(84)90028-5.
Lischeid, G. & T. Kalettka, 2012. Grasping the heterogeneity of kettle hole water quality in Northeast Germany. Hydrobiologia 689: 63–77. https://doi.org/10.1007/s10750-011-0764-7.
Liu, J., J. Soininen, B. Han & S. A. Declerck, 2013. Effects of connectivity, dispersal directionality and functional traits on the metacommunity structure of river benthic diatoms. Journal of Biogeography 40: 2238–2248. https://doi.org/10.1111/jbi.12160.
Lopes, P. M., R. Bozelli, L. M. Bini, J. M. Santangelo & S. A. Declerck, 2016. Contributions of airborne dispersal and dormant propagule recruitment to the assembly of rotifer and crustacean zooplankton communities in temporary ponds. Freshwater Biology 61: 658–669. https://doi.org/10.1111/fwb.12735.
Louette, G. & L. De Meester, 2004. Rapid colonization of a newly created habitat by cladocerans and the initial build-up of a Daphnia-dominated community. Hydrobiologia 513: 245–249. https://doi.org/10.1023/B:hydr.0000018299.54922.57.
Louette, G., L. De Meester & S. Declerck, 2008. Assembly of zooplankton communities in newly created ponds. Freshwater Biology 53: 2309–2320. https://doi.org/10.1111/j.1365-2427.2008.02052.x.
Lundberg, J. & F. Moberg, 2003. Mobile link organisms and ecosystem functioning: implications for ecosystem resilience and management. Ecosystems 6: 0087–0098. https://doi.org/10.1007/s10021-002-0150-4.
Maguire, B., 1963. The passive dispersal of small aquatic organisms and their colonization of isolated bodies of water. Ecological Monographs 33: 161–185. https://doi.org/10.2307/1948560.
Microsoft Corporation. 2016. Microsoft Excel. Retrieved from https://office.microsoft.com/excel
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. O’hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, & H. Wagner, 2022. Package ‘vegan. Community Ecology Package. R package version 2.5-6. http://CRAN.Rproject.org/package=vegan.
Onandia, G., S. Maassen, C. L. Musseau, S. A. Berger, C. Olmo, J. M. Jeschke & G. Lischeid, 2021. Key drivers structuring rotifer communities in ponds: insights into an agricultural landscape. Journal of Plankton Research 43: 396–412. https://doi.org/10.1093/plankt/fbab033.
Pinceel, T., L. Brendonck & B. Vanschoenwinkel, 2016. Propagule size and shape may promote local wind dispersal in freshwater zooplankton – a wind tunnel experiment. Limnology and Oceanography 61: 122–131. https://doi.org/10.1002/lno.10201.
R Core Team, 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.r-project.org/.
Santangelo, J. M., P. M. Lopes, M. O. Nascimento, A. P. C. Fernandes, S. Bartole, M. P. Figueiredo-Barros, J. J. Leal, F. A. Esteves, V. F. Farjalla & C. C. Bonecker, 2015. Community structure of resting egg banks and concordance patterns between dormant and active zooplankters in tropical lakes. Hydrobiologia 758: 183–195. https://doi.org/10.1007/s10750-015-2289-y.
Schlägel, U. E., V. Grimm, N. Blaum, P. Colangeli, M. Dammhahn, J. A. Eccard, S. L. Hausmann, A. Herde, H. Hofer, J. Joshi, S. Kramer-Schadt, M. Litwin, S. D. Lozada-Gobilard, M. E. H. Mueller, T. Mueller, R. Nathan, J. S. Petermann, K. Pirhofer-Walzl, V. Radchuk, M. C. Rillig, M. Roeleke, M. Schaefer, C. Scherer, G. Schiro, C. Scholz, L. Teckentrup, R. Tiedemann, W. Ullmann, C. C. Voigt, G. Weithoff & F. Jeltsch, 2020. Movement-mediated community assembly and coexistence. Biological Reviews 95(4): 1073–1096. https://doi.org/10.1111/brv.12600.
Sciullo, L., & J. Kolasa, J, 2012. Linking local community structure to the dispersal of aquatic invertebrate species in a rock pool metacommunity. Community Ecology 13:203–212. https://doi.org/10.1556/ComEc.13.2012.2.10.
Serrano L., M. Reina, X. D. Quintana, S. Romo, C. Olmo, J.M. Soria, S. Blanco, C. Fernández-Aláez, M. Fernández-Aláez, M.C. Caria, S. Bagella, T. Kalettka, M. Pätzig, 2017. A new tool for the assessment of severe anthropogenic eutrophication in small shallow water bodies. Ecological Indicators 76: 324–334. https://doi.org/10.1016/j.ecolind.2017.01.034.
Sih, A., P. Crowley, M. McPeek, J. Petranka & K. Strohmeier, 1985. Predation, competition, and prey communities: a review of field experiments. Annual Review of Ecology and Systematics 16: 269–311. https://doi.org/10.1146/annurev.es.16.110185.001413.
Soininen, J., M. Kokocinski, S. Estlander, J. Kotanen & J. Heino, 2007. Neutrality, niches, and determinants of plankton metacommunity structure across boreal wetland ponds. Ecoscience 14: 146–154. https://doi.org/10.2980/1195-6860(2007)14[146:NNADOP]2.0.CO;2.
Vanschoenwinkel, B., A. Waterkeyn, T. Nhiwatiwa, T. Pinceel, E. Spooren, A. Geerts, B. Clegg & L. Brendonck, 2011. Passive external transport of freshwater invertebrates by elephant and other mud-wallowing mammals in an African savannah habitat. Freshwater Biology 56: 1606–1619. https://doi.org/10.1111/j.1365-2427.2011.02600.x.
Vanschoenwinkel, B., C. De Vries, M. Seaman & L. Brendonck, 2007. The role of metacommunity processes in shaping invertebrate rock pool communities along a dispersal gradient. Oikos 116: 1255–1266. https://doi.org/10.1111/j.0030-1299.2007.15860.x.
Vanschoenwinkel, B., A. Waterkeyn, T. Vandecaetsbeek, O. Pineau, P. Grillas & L. Brendonck, 2008a. Dispersal of freshwater invertebrates by large terrestrial mammals: a case study with wild boar (Sus scrofa) in Mediterranean wetlands. Freshwater Biology 53: 2264–2273. https://doi.org/10.1111/j.1365-2427.2008.02071.x.
Vanschoenwinkel, B., S. Gielen, H. Vandewaerde, M. Seaman & L. Brendonck, 2008b. Relative importance of different dispersal vectors for small aquatic invertebrates in a rock pool metacommunity. Ecography 31: 567–577. https://doi.org/10.1111/j.0906-7590.2008.05442.x.
Vanschoenwinkel, B., S. Gielen, M. Seaman & L. Brendonck, 2008c. Any way the wind blows-frequent wind dispersal drives species sorting in ephemeral aquatic communities. Oikos 117: 125–134. https://doi.org/10.1111/j.2007.0030-1299.16349.x.
Vanschoenwinkel, B., S. Gielen, M. Seaman & L. Brendonck, 2009. Wind mediated dispersal of freshwater invertebrates in a rock pool metacommunity: differences in dispersal capacities and modes. Hydrobiologia 635: 363–372. https://doi.org/10.1007/s10750-009-9929-z.
Voigt, M., & W. Koste, 1978. Rotatoria: die Rädertiere Mitteleuropas; ein Bestimmungswerk; Überordnung Monogononta. 1. Textband. Borntraeger.
Waterkeyn, A., P. Grillas, B. Vanschoenwinkel & L. Brendonck, 2008. Invertebrate community patterns in Mediterranean temporary wetlands along hydroperiod and salinity gradients. Freshwater Biology 53: 1808–1822. https://doi.org/10.1111/j.1365-2427.2008.02005.x.
Waterkeyn, A., B. Vanschoenwinkel, P. Grillas & L. Brendoncka, 2010. Effect of salinity on seasonal community patterns of Mediterranean temporary wetland crustaceans: a mesocosm study. Limnology and Oceanography 55: 1712–1722. https://doi.org/10.4319/lo.2010.55.4.1712.
Wilson, D. S., 1992. Complex interactions in metacommunities, with implications for biodiversity and higher levels of selection. Ecology 73: 1984–2000. https://doi.org/10.2307/1941449.
Zuur, A. F., E. N. Ieno, N. J. Walker, A. A. Saveliev, & G. M. Smith, 2009. Mixed effects models and extensions in ecology with R. Springer, 574. https://doi.org/10.1007/978-0-387-87458-6
Acknowledgements
We thank all members of the BioMove research training group for helpful discussions. We thank the farmers and landowners for their cooperation in permitting the sampling of the ponds. We also thank Maxi Tomowski, Jonas Stiegler and Dominique C. Noetzel for excellent assistance during fieldwork. We also thank Michael Ristow for aquatic macrophytes identification.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by the Deutsche Forschungsgemeinschaft (DFG), BioMove research training group (www.biomove.org/), Grant No. GRK 2118.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, investigation, VP, RT, and GW; writing—original draft preparation, VP; writing—review and editing, VP, KK, JP, RT and GW; supervision, GW, JE and RT; funding acquisition, GW, JE and RT. All authors have read and provided extensive comments on the manuscript concerning analysis and interpretation. All authors have agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Maria Špoljar, Diego Fontaneto, Elizabeth J. Walsh & Natalia Kuczyńska-Kippen / Diverse Rotifers in Diverse Ecosystems
Supplementary Information
Below is the link to the electronic supplementary material.
SUPPLEMENTARY INFORMATION
The following supporting information can be downloaded at: www. / /. Table S1 Aquatic plants found in mesocosms. Table S2 Zooplankton species found in mesocosms. Table S3 Pairwise comparisons. Fig. S1 Trophi identification of species. a) Epiphanes brachionus b) Brachionus quadridentatus c) Trichocerca brachyura d) Lecane bulla. Fig.S2 Biotic vectors caught on camera. a) Roe deer b) Raccoon c) Wild boar d) White Stork. Fig. S3 QQplot of model residuals showing normality for rotifers. Fig. S4 QQplot of model residuals showing normality for cladocerans. Fig. S5 Stress plots of NMDS analyses based on species community composition of rotifers for each of the sampling period. Fig. S6 Stress plots of NMDS analyses based on species community composition of cladocerans for each of the sampling period. (DOCX 2006 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parry, V., Kiemel, K., Pawlak, J. et al. Drivers of zooplankton dispersal in a pond metacommunity. Hydrobiologia 851, 2875–2893 (2024). https://doi.org/10.1007/s10750-023-05232-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05232-4