Abstract
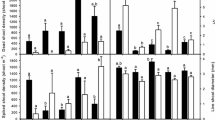
Phenotypic plasticity of the two salt marsh grasses Spartina alterniflora and Phragmites australis in salt marshes is crucial to their invasive ability, but the importance of phenotypic plasticity, nitrogen levels, and intraspecific competition to the success of the two species is unclear at present. Spartina alterniflora Loisel. is an extensively invasive species that has increased dramatically in distribution and abundance on the Chinese and European coasts, and has had considerable ecological impacts in the regions where it has established. Meanwhile, Phragmites australis Cav., a native salt marsh species on the east coast of China, has replaced the native S. alterniflora in many marshes along the Atlantic Coast of the US. This study determined the effects of nitrogen availability and culm density on the morphology, growth, and biomass allocation traits of Spartina alterniflora and Phragmites australis. A large number of morphological, growth, and biomass parameters were measured, and various derived values (culm: root ratio, specific leaf area, etc.) were calculated, along with an index of phenotypic plasticity. Nitrogen addition significantly affected growth performance and biomass allocation traits of Spartina alterniflora, and culm density significantly affected morphological characteristics in a negative way, especially for Spartina alterniflora. However, there were no significant interactions between nitrogen levels and culm density on the morphological parameters, growth performances parameters, and biomass allocation parameters of the two species. Spartina alterniflora appears to respond more strongly to nitrogen than to culm density and this pattern of phenotypic plasticity appears to offer an expedition for successful invasion and displacement of Phramites australias in China. The implication of this study is that, in response to the environmental changes that are increasing nitrogen levels, the range of Spartina alterniflora is expected to continue to expand on the east coast of China.






Similar content being viewed by others
References
Avramov, S., D. Pemac & B. Tucic, 2007. Phenotypic plasticity in response to an irradiance gradient in Iris pumila: adaptive value and evolutionary constraints. Plant Ecology 190: 275–290.
Callaway, R. M., S. C. Pennings & C. L. Richards, 2003. Phenotypic plasticity and interactions among plants. Ecology 84: 1115–1128.
Chen, X. Y., 2000. Effects of plant density and age on the mating system of Kandelia candel Druce (Rhizophoraceae), a viviparous mangrove species. Hydrobiologia 432: 189–193.
Cipollini, D. F. & J. Bergelson, 2001. Plant density and nutrient availability constrain constitutive and wound-induced expression of trypsin inhibiters in Brassica napus. Journal of Chemical Ecology 27: 593–610.
Cloern, J. E., 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210: 223–253.
Daehler, C. C., 2003. Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annual Review of Ecology, Evolution, and Systematics 34: 183–211.
Dukes, J. S. & H. A. Mooney, 1999. Does global change increase the success of biological invaders? Trends Ecology and Evolution 14: 135–139.
Elberse, I. A. M. & J. M. Damme, 2003. Plasticity of growth characteristics in wild barley (Hordeum spontaneum) in response to nutrient limitation. Journal of Ecology 91: 371–382.
Farnsworth, E. J. & L. A. Meyerson, 2003. Comparative ecophysiology of four wetland plant species along a continuum of invasiveness. Wetlands 23: 750–762.
Fransen, B. H., D. Kroon & F. Berendse, 1998. Root morphological plasticity and nutrient acquisition of perennial grass species from habitats of different nutrient availability. Oecologia 115: 351–358.
Funk, J. L., 2008. Differences in plasticity between invasive and native plants from a low resource environment. Journal of Ecology 96: 1162–1173.
Grace, J. B. & B. H. Pugesek, 1997. A structural equation model of plant species richness and its application to a coastal wetland. American Naturalist 149: 436–460.
Grotkopp, E., M. Rejmánek & T. L. Rost, 2002. Toward a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. American Naturalist 159: 396–419.
Hemminga, M. A. & G. J. C. Buth, 1991. Decomposition in saltmarsh ecosystems of the Sw Netherlands–the effects of biotic and abiotic factors. Vegetatio 92: 73–83.
Hierro, J. L., L. M. John & R. M. Callaway, 2005. A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. Journal of Ecology 93: 5–15.
Idestam-Almquist, J. & L. Kautsky, 1995. Plastic responses in morphology of Potamogeton pectinatus L. to sediment condition at two sites in the northern Baltic proper. Aquatic Botany 52: 205–216.
Jean, H. B. & A. W. Alice, 2006. A comparison of plastic responses to competition by invasive and non-invasive congeners in the Commelinaceae. Biological Invasions 8: 797–807.
Li, M. T., K. Q. Xu, M. Watanabe & Z. Y. Chen, 2007. Long-term variations in dissolved silicate, nitrogen, and phosphorus flux from the Yangtze River into the East China Sea and impacts on estuarine ecosystem. Estuarine Coastal and Shelf Science 71: 3–12.
Maherali, H. & E. H. DeLucia, 2001. Influence of climate-driven shifts in biomass allocation on water transport and storage in ponderosa pine. Oecologia 129: 481–489.
Marks, M., B. Lapin & J. Randall, 1994. Phragmites australis (P. communis): threats, management, and monitoring. Natural Areas Journal 14: 285–294.
Nagashima, H. & I. Terashima, 1995. Effects of plant density on frequency distributions of plant height in Chenopodium album stands: analysis based on continuous monitoring of height-growth of individual plants. Annals of Botany (London) 75: 173–180.
Perez, M., C. M. Duarte, J. Romero, K. Sand-Jensen & T. Alcoverro, 1994. Growth plasticity in Cymodocen nodosa stands: the importance of nutrient supply. Aquatic Botany 47: 249–264.
Poorter, L., 1999. Growth responese of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Functional Ecology 13: 396–410.
Poulin, B., G. Lefebvre & A. Mauchamp, 2002. Habitat requirements of passerines and phragmites australis bed management in Southern France. Biological Conservation 107: 315–325.
Ryser, P. & H. Lambers, 1995. Root and leaf attribute accounting for the performance of fast- and slow-growing grasses at different nutrient supply. Plant and Soil 170: 251–265.
Schlichting, C. D., 1986. The evolution of phenotypic plasticity in plant. Annual Review of Ecology and Systematics 17: 667–693.
Shen, Z. L., Q. Liu, S. M. Zhang, H. Miao & P. Zhang, 2001. The dominant controlling factors of high content inorganic N in the Changjing River and its mouth. Oceanologia et Limnologia Sinica 32: 465–473.
Tyler, A. C., J. G. Lambrinos & E. D. Grosholz, 2007. Nitrogen inputs promote the spread of an invasive marsh grass. Ecological Applications 17: 1886–1898.
Valladares, F., S. J. Wright, E. Lasso, K. Kitajima & R. W. Pearcy, 2000. Plastic phenotypic response to light of 16 congeneric shrubs from a panamanian rainforest. Ecology 81: 1925–1936.
Vasquez, E. A., E. P. Glenn, G. R. Guntenspergen & J. J. Brown, 2006. Salt tolerance and osmotic adjustment of Spartina alterniflora (Poaceae) and the invasive M haplotype of Phragmites australis (Poaceae) along a salinity gradient. American Journal of Botany 93: 1784–1790.
Vitousek, P. M., 1986. Biological invasions and ecosystem properties: can species make a difference? Ecological Studies 58: 163–176.
Wang, B. D., 2006. Cultural eutrophication in the Changjing (Yangtze River) plume: History and perspective. Estuarine, Coastal and Shelf Science 69: 471–477.
Wang, J. F. & Y. L. Feng, 2004. The effect of light intensity on biomass allocation, leaf morphology and relative growth rate of two invasive plants. Acta Phytoecologica Sinica 28: 781–786 (in Chinese with English abstract).
Wang, Q., C. H. Wang, B. Zhao, Z. J. Ma, Y. Q. Luo, J. K. Chen & B. Li, 2006. Effects of growing conditions on the growth of and interactions between salt marsh plants: implications for invasibility of habitats. Biological Invasions 8: 1547–1560.
Williams, D. G. & R. A. Black, 1994. Drought response of a native and introduced Hawaiian grass. Oecologia 97: 512–519.
Wilsey, B. & H. W. Polley, 2006. Aboveground productivity and root-shoot allocation differ between native and introduced grass species. Oecologia 150: 300–309.
Windham, L. & R. G. Lathrop, 1999. Effects of Phragmites australis invasion on aboveground biomass and soil properties in brackish tidal marsh of the Mullica River, New Jersey. Estuaries 22: 927–935.
Zhao, C. J., Z. F. Deng, C. F. Zhou, B. H. Guan & S. Q. An, 2008. Effects of nitrogen availability and competition on leaf characteristics of Spartina alterniflora and Phragmite australis. Journal of Plant Ecology 32: 392–401. (in Chinese with English abstract).
Zheng, Y. L., Y. L. Feng, W. X. Liu & Z. Y. Liao, 2009. Growth, biomass allocation, morphology, and photosynthesis of invasive Eupatorium adenophorum and its native congeners grown at four irradiances. Plant Ecology 203: 263–271.
Zhu, Z. H., J. X. Liu & X. A. Wang, 2007. Review of phenotypic plasticity and hierarchical selection in clonal plants. Jonrnal of Plant Ecology (Chinese Version) 31: 588–598. (in Chinese with English abstract).
Acknowledgement
This study is financially supported by the Special Research Program for Public- welfare Forestry (200804005), the Natural Scientific Foundation of Jiangsu Province, China (BK2009154), the National Natural Science Foundation of China (No. 30770358 and 30700061), the Fund of Post-doctor in Jangshu Province (0901017C) and Natural Science Foundation of Jiangsu Province (BK 2007152). The authors thank the colleagues and students from Nanjing University for helping with field and lab experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yong jun Zhao and Hua Qing are the co-first authors.
Handling editor: S. A. Halse
Rights and permissions
About this article
Cite this article
Zhao, Y.j., Qing, H., Zhao, C.j. et al. Phenotypic plasticity of Spartina alterniflora and Phragmites australis in response to nitrogen addition and intraspecific competition. Hydrobiologia 637, 143–155 (2010). https://doi.org/10.1007/s10750-009-9992-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-9992-5