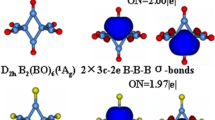
Abstract
Many pictures of the bonding of B2H6 have been presented over the past century, starting with a strong effort to force B2H6 to fit the ideas that were current for C2H6 and building to now viewing the molecular orbital model as the basis for a new transferrable concept of a 3-center-2-electron bond that stimulates creation of new chemistry. Even now, though, some would view this special bond more like a protonated double bond. The historical development of the current understanding of bonding in B2H6 is summarized here.



Similar content being viewed by others
References
Bauer, S.H.: The structure of diborane. J. Amer. Chem. Soc. 59, 1096–1103 (1937)
Bauer, S.H.: The structure of the hydrides of boron. V. tetraborane and the pentaborane B5H11. J. Amer. Chem. Soc. 60, 805–812 (1938)
Bauer, S.H.: Structures and physical properties of the hydrides of boron and of their derivatives. Chem. Rev. 31, 43–75 (1942)
Brown, G.I.: A Simple Guide to Modern Valency Theory. Longmans, London (1953)
Brundle, C.R., Robin, M.B., Basch, H., Pinsky, M., Bond, A.: Experimental and theoretical comparison of the electronic structures of ethylene and diborane. J. Amer. Chem. Soc. 92, 3862–3866 (1970)
Core, A.F.: Chemical combination and the constitution of boron hydride. J. Soc. Chem. Indus. 46, 642–643 (1927)
Dilthey, W.: Über die Konstitution des Wassers. Z. Für Angew. Chem. 34, 596 (1921)
Eastman, E.D.: Double and triple bonds, and electron structures in unsaturated molecules. J. Amer. Chem. Soc. 44, 438–451 (1922)
Eaton, G. R. and Lipscomb, W. N.: N.M.R. Studies of Boron Hydrides and Related Compounds (1969)
Eberhardt, W.H., Crawford, J.B., Lipscomb, W.N.: The valence structure of the boron hydrides. J. Chem. Phys. 22, 989–1001 (1954)
Förster, D., Hübschle, C.B., Lugar, P., Hügle, T., Lentz, D.: On the 2-electron 3-center bond: charge density determination of tetraborane (10). Inorg. Chem. 47, 1874–1876 (2008)
Gambaryan, N.P., Stankevich, I.V.: The development of the concept of the chemical bond from hydrogen to cluster compounds. Russ. Chem. Rev. 58, 1103–1118 (1989)
Goebbert, D.J., Hernandez, H., Francisco, J.S., Wenthold, P.G.: The binding energy and bonding in dialane. J. Amer. Chem. Soc. 127, 11684–11689 (2005)
Harris, R.M., Zhu, Z., Deepika, A., Tufekci, B.A., Peterson, K., Jena, P., Bowen, K.H.: Au as a surrogate for F: the case of UAu6 vs UF6. J. Amer. Chem. Soc. 1444, 19685–19688 (2022)
Hedberg, K., Schomaker, V.: A reinvestigation of diborane and ethane by electron diffraction. J. Amer. Chem. Soc. 73, 1482–1487 (1951)
Holleman, A.F., Cooper, H.C.: A Text-Book of Inorganic Chemistry, in English, 2nd edn. NY, Wiley (1905)
Holleman, A.F., Cooper, H.C.: A Text-Book of Inorganic Chemistry, in English, 7th edn. John Wiley, N. Y. (1927)
Housecroft, C.E., Sharpe, A.G.: Inorganic Chemistry, 5th edn. Harlow, Pearson (2018)
Hückel, W.: Structural Chemistry of Inorganic Compounds, pp. 494–495. Elsevier Publishing Company, Amsterdam (1951)
Laszlo, P.: A diborane story. Angew. Chem. Int. Ed. 39, 2071–2072 (2000)
Latimer, W.M., Hildebrand, J.H.: Reference Book of Inorganic Chemistry. The MacMillan Company, New York (1940)
Latimer, W.M., Hildebrand, J.H.: Reference Book of Inorganic Chemistry, 3rd edn. The MacMillan Company, New York (1951)
Lewis, G.N.: Introductory address: valence and the electron. Trans. Faraday Soc. 19, 452–458 (1923a)
Lewis, G.N.: The chemical bond. J. Chem. Phys. 1, 17–28 (1933)
Lewis, G.N.: Valence and The Structure of Atoms and Molecules. The Chemical Catalog Company, New York (1923)
Lipscomb, W.N.: Valence in the boron hydrides. J. Chem. Phys. 61, 23–27 (1957)
Lipscomb, W.N.: Boron Hydrides. Benjamin Inc, N. Y., W. A (1963)
Lipscomb, W.N., Kaczmarczyk, A.: Interactions of boranes and their ions with paramagnetic S-state ions. Proc. Natl. Acad. Sci. u. s. 47, 1796–1797 (1961)
Longuet-Higgins, H. C. and Bell, R. P.: The structure of the boron hydrides. J. Chem. Soc.: 250–255 (1943)
Longuet-Higgins, H. C.: The structure of some electron-deficient molecules. J. Chem. Soc.: 139–143 (1946)
Mulliken, R.S.: Electronic structures of molecules. XIII. Diborane and related molecules. J. Chem. Phys. 3, 635–645 (1935)
Mulliken, R.S.: The structure of diborane and related molecules. Chem. Rev. 41, 207–217 (1947)
Parkin, G.: Representation of three-center-two-electron bonds in covalent molecules with bridging hydrogen atoms. J. Chem. Ed. 96, 2467–2475 (2019)
Partington, J.R.: General and Inorganic Chemistry for University Students. Macmillan and Co, London (1949)
Partington, J.R.: General and Inorganic Chemistry for University Students, 3rd edn. New York, Macmillan and Co (1958)
Pauling, L.: The nature of the chemical bond. II. The one-electron bond and the three-electron bond. J. Amer. Chem. Soc. 53, 3225–3237 (1931)
Pauling, L.: The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry, 3rd edn. Cornell University Press, Ithaca (1960)
Pitzer, K.S.: Electron deficient molecules. I. The principles of hydroboron structures. J. Amer. Chem. Soc. 67, 1126–1132 (1945)
Price, W.C.: The structure of diborane. J. Chem. Phys. 15, 614 (1947)
Robinson, R.: Intramolecular ionisation. Trans. Faraday Soc. 18, 295–301 (1923)
Rundle, R.E.: Electron deficient compounds. J. Amer. Chem. Soc. 69, 1327–1331 (1947)
Rundle, R.E.: Electron deficient compounds. II. Relative energies of “half-bonds.” J. Chem. Phys. 17, 671–675 (1949)
Schlesinger, H.I., Burg, A.B.: Recent developments in the chemistry of the boron hydrides. Chem. Rev. 31, 1–41 (1942)
Shoolery, J.N.: The relation of high resolution nuclear magnetic resonance spectra to molecular structures. Disc. Faraday Soc. 19, 215–225 (1955)
Sidgwick, N.V.: The nature of the non-polar link. Trans. Faraday Soc. 19, 469–475 (1923)
Sidgwick, N.V.: The Electronic Theory of Valency. Oxford University Press, Oxford (1932)
Sidgwick, N.V.: The Chemical Elements and Their Compounds. Oxford, Oxford (1950)
Smith, J.D.M.: Chemistry & Atomic Structure. Van Nostrand, N. Y. (1924)
Smith, J.D.M.: Constitutions of the hydroborons. Chem. News J. Indus. Sci 135, 81–88 (1927)
Stock, A.: Hydrides of Boron and Silicon. Cornell University Press, Ithaca (1933)
International Library of Technology.: Inorganic Chemistry. International Textbook Company, London (1905)
Thomson, J.J.: Discussion on part I. Trans. Faraday Soc. 19, 476 (1923)
Walsh, A. D.: Co-ordinate links formed by bonding electrons: a suggestion regarding the structure of diborane. J. Chem. Soc.: 89 – 92 (1947)
Acknowledgements
This work was supported in part by the University of Denver. My introduction to the bonding of the boron hydrides was in the laboratory of W. N. Lipscomb in 1961-1962 and 1967, see (Eaton and Lipscomb 1969).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eaton, G.R. Interpreting the bonding of B2H6 and the nature of the 3-center-2-electron bond: decisive test of theory of valency. Found Chem 25, 285–298 (2023). https://doi.org/10.1007/s10698-023-09469-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10698-023-09469-8