Abstract
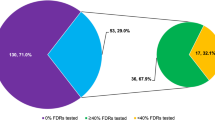
Cascade testing for familial cancer syndromes has historically been difficult to execute. As part of a facilitated cascade testing pathway, we evaluated barriers to completion of cascade testing. Our previously published study evaluated a facilitated cascade testing pathway whereby a genetics team facilitated at-risk relative (ARR) cascade testing through telephone genetic counseling and mailed saliva kit testing. This follow-up study evaluated barriers to completion of cascade genetic testing through six-month follow-up telephone interviews. Probands identified 114 ARRs, of whom 97 were successfully contacted by telephone. Among those contacted, 83 (86%) reported interest in genetic testing and 14 (14%) declined. Among those reporting interest in testing, 71% (69/83) completed testing. Follow-up telephone interviews revealed that 14 ARRs did not complete testing despite reporting interest for the following reasons: concern about genetic discrimination, fear of a positive result and belief that the pathogenic variant was not relevant to his/her health. Five ARRs reported that they remained interested in testing and the telephone call prompted completion of testing. Even when facilitated by a medical team with prioritization of relative convenience, significant barriers to cascade testing ARRs for hereditary breast and ovarian cancer syndrome persist due to concern about genetic discrimination, cost, and fear of positive test results.

Similar content being viewed by others
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Drohan B, Roche CA, Cusack JC, Hughes KS (2012) Hereditary breast and ovarian cancer and other hereditary syndromes: using technology to identify carriers. Ann Surg Oncol Jun 19(6):1732–1737. doi:https://doi.org/10.1245/s10434-012-2257-y
Practice CoG. ACOG Committee Opinion No (2018) ;131(1):e31-e34. doi:https://doi.org/10.1097/AOG.0000000000002457
Randall LM, Pothuri B, Swisher EM et al (2017) Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol 08(2):217–224. doi:https://doi.org/10.1016/j.ygyno.2017.06.002
Offit K, Tkachuk KA, Stadler ZK et al (2020) Cascading After Peridiagnostic Cancer Genetic Testing: An Alternative to Population-Based Screening. J Clin Oncol Jan JCO1902010. doi:https://doi.org/10.1200/JCO.19.02010
Offit K (2016) The future of clinical cancer genomics. Semin Oncol Oct 43(5):615–622. doi:https://doi.org/10.1053/j.seminoncol.2016.10.002
Finch AP, Lubinski J, Møller P et al (2014) Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol May 32(15):1547–1553. doi:https://doi.org/10.1200/JCO.2013.53.2820
Daly MB et al (2022) NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment:Breast, Ovarian, and Pancreatic, Version 2.2022. J Natl Compr Canc Netw
Blandy C, Chabal F, Stoppa-Lyonnet D, Julian-Reynier C (2003) Testing participation in BRCA1/2-positive families: initiator role of index cases. Genetic Test Fall 7(3):225–233. doi:https://doi.org/10.1089/109065703322537241
Fehniger J, Lin F, Beattie MS, Joseph G, Kaplan C (2013) Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J Genet Couns Oct 22(5):603–612. doi:https://doi.org/10.1007/s10897-013-9592-4
Hafertepen L, Pastorino A, Morman N et al (2017) Barriers to genetic testing in newly diagnosed breast cancer patients: Do surgeons limit testing? Am J Surg 214(1):105–110
Hinchcliff EM, Bednar EM, Lu KH, Rauh-hain JA (2019) Disparities in gynecologic cancer genetics evaluation. Gynecol Oncol 153(1):184–191
Sheppard VB, Mays D, LaVeist T, Tercyak KP (2013) Medical mistrust influences black women’s level of engagement in BRCA 1/2 genetic counseling and testing. J Natl Med Assoc 105(1):17–22
Forman AD, Hall MJ (2009) Influence of race/ethnicity on genetic counseling and testing for hereditary breast and ovarian cancer. Breast J 15(Suppl 1):S56–62
Ford ME, Alford SH, Britton D, Mcclary B, Gordon HS (2007) Factors influencing perceptions of breast cancer genetic counseling among women in an urban health care system. J Genet Couns 16(6):735–753
Frey MK, Kahn RM, Chapman-davis E et al(2020) Prospective Feasibility Trial of a Novel Strategy of Facilitated Cascade Genetic Testing Using Telephone Counseling.J Clin Oncol. ;:JCO1902005
George R, Kovak K, Cox SL (2015) Aligning policy to promote cascade genetic screening for prevention and early diagnosis of heritable diseases. J Genet Couns Jun 24(3):388–399. doi:https://doi.org/10.1007/s10897-014-9805-5
Walsh T, Casadei S, Lee MK et al (2011) Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A Nov 108(44):18032–18037. doi:https://doi.org/10.1073/pnas.1115052108
Lu KH, Wood ME, Daniels M et al (2014) American Society of Clinical Oncology Expert Statement: collection and use of a cancer family history for oncology providers. J Clin Oncol Mar 32(8):833–840. doi:https://doi.org/10.1200/JCO.2013.50.9257
Committee on Practice Bulletins–Gynecology CoG, S.ciety of Gynecologic Oncology (2017) ;130(3):e110-e126. doi:https://doi.org/10.1097/AOG.0000000000002296
Konstantinopoulos PA, Norquist B, Lacchetti C et al(2020) Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline.J Clin Oncol. ;:JCO1902960
Katapodi MC, Viassolo V, Caiata-Zufferey M et al (2017) Cancer Predisposition Cascade Screening for Hereditary Breast/Ovarian Cancer and Lynch Syndromes in Switzerland: Study Protocol. JMIR Res Protoc Sep 6(9):e184. doi:https://doi.org/10.2196/resprot.8138
Suthers GK, Armstrong J, McCormack J, Trott D (2006) Letting the family know: balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J Med Genet Aug 43(8):665–670. doi:https://doi.org/10.1136/jmg.2005.039172
Sharaf RN, Myer P, Stave CD, Diamond LC, Ladabaum U (2013) Uptake of genetic testing by relatives of lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol Sep 11(9):1093–1100. doi:https://doi.org/10.1016/j.cgh.2013.04.044
Lieberman S, Lahad A, Tomer A et al (2018) Familial communication and cascade testing among relatives of BRCA population screening participants. Genet Med 20(11):1446–1454
Narod SA, Butler R, Bobrowski D et al (2018) Short report: Follow-up of Bahamian women with a BRCA1 or BRCA2 mutation. Mol Genet Genomic Med 6(2):301–304
Provenzale D et al (2019) Genetic/Familial High-Risk Assessment: Colorectal Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw
Donenberg T, George S, Ali J et al (2019) A clinically structured and partnered approach to genetic testing in Trinidadian women with breast cancer and their families. Breast Cancer Res Treat 174(2):469–477
MacDonald DJ, Blazer KR, Weitzel JN (2010) Extending com- prehensive cancer center expertise in clinical cancer genetics and genomics to diverse communities: the power of partnership. J Natl Compr Cancer Netw 8(5):615–624
Acknowledgements of research support
This project was supported by the Weill Cornell Medicine Clinical & Translational Science Center (CTSC) and Invitae Genetics. Paul Christos was partially supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000457. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
There are no sources of funding for the research reported.
Author information
Authors and Affiliations
Contributions
Conception and design: Melissa K. Frey, Francesca Tubito, Ryan Kahn, Kevin Holcomb. Financial support: Paul Christos. Administrative support: Francesca Tubito. Provision of study materials or patients: Francesca Tubito, Ryan Kahn. Collection and assembly of data: Melissa K. Frey, Ryan Kahn, Eloise Chapman-Davis, Maira Pires, Samantha Anderson, Thomas A. Caputo. Data analysis and interpretation: Melissa K. Frey, Ryan Kahn, Paul Christos, Ravi N. Sharaf, Kenneth Offit, Kevin Holcomb, Hannah Krinsky, Steven Lipkin, Roni Nitecki, Jose Alejandro Rauh-Hain, Rana Khan Fowlkes. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Corresponding author
Ethics declarations
Ethics approval
Approved by Weill Cornell Medicine Institutional Review Board. Our study was reviewed by an institutional review board/human investigations committee/ethics committee (Weill Cornell Institutional Review Board) and approved as human subjects research.
Human studies and informed consent
All appropriate steps were taken in obtaining informed consent of all human subjects participating in the research comprising the manuscript submitted for publication. There is no identifying information. All study participants have given informed consent to be included in the study.
Statements and declarations
Kevin Holcomb serves as a consultant for Johnson and Johnson and receives research support from Fujirebio Diagnostics. None of the remaining authors have a conflict of interest to disclose.
Conflict of interest / competing interests
Ryan M. Kahn declares that he has no conflict of interest. Muhammad Danyal Ahsan declares that he has no conflict of interest. Eloise Chapman-Davis declares that she has no conflict of interest. Kevin Holcomb declares that he has no conflict of interest. Roni Nitecki declares that she has no conflict of interest. Jose Alejandro Rauh-Hain declares that he has no conflict of interest. Rana Khan Fowlkes declares that she has no conflict of interest. Francesca Tubito declares that she has no conflict of interest. Maira Pires declares that she has no conflict of interest. Paul J Christos declares that he has no conflict of interest. Kaitlyn Tkachuk declares that she has no conflict of interest. Hannah Krinsky declares that she has no conflict of interest. Ravi N. Sharaf declares that he has no conflict of interest. Kenneth Offit declares that he has no conflict of interest. Steven Lipkin declares that he has no conflict of interest. Melissa K. Frey declares that she has no conflict of interest.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kahn, R.M., Ahsan, M.D., Chapman-Davis, E. et al. Barriers to completion of cascade genetic testing: how can we improve the uptake of testing for hereditary breast and ovarian cancer syndrome?. Familial Cancer 22, 127–133 (2023). https://doi.org/10.1007/s10689-022-00316-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-022-00316-x