Abstract
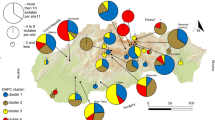
Dothistroma needle blight (DNB) is regarded as one of the most significant foliar diseases of pine and has caused significant damage to pine plantations in France since the late 1990s. The genetic diversity, reproductive strategy and population structure of D. septosporum, one of the causal agents of DNB, was investigated at six sites across Brittany, France, using species-specific mating type primers and microsatellite markers. Multiplex PCR assays were developed to effectively and efficiently screen 11 of the available microsatellite markers on the isolates obtained. From 282 isolates analysed, 81 unique multilocus haplotypes were identified, indicating a diverse (haplotypic diversity ranged from 0.403 to 0.962) yet highly clonal structure of the pathogen at these sites. Although all sites contained both mating types, random mating was rejected by the index of association and parsimony tree length permutation tests on both clone-corrected and non-clone-corrected datasets but supported by mating type ratios in the clone-corrected datasets. Four population clusters were identified using STRUCTURE. Neither locality nor host species played a significant role in the population structure in Brittany. These findings, along with the observation of identical haplotypes at different sites and on different hosts, suggest movement of the pathogen within the peninsula. Overall, the pathogen’s population structure was found to be influenced by sexual recombination yet within sites clonal reproduction predominated.




Similar content being viewed by others
References
Agapow, P. M., & Burt, A. (2001). Indices of multilocus linkage disequilibrium. Molecular Ecology Notes, 1(1–2), 101–102.
Aumonier, T. (2002). La maladie des bandes rouges toujours en augmentation en 2001. Bilan de la santé des forêts, 2001, 58–60.
Bandelt, H. J., Forster, P., & Röhl, A. (1999). Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution, 16, 37–48.
Barnes, I., Crous, P. W., Wingfield, B. D., & Wingfield, M. J. (2004). Multigene phylogenies reveal that red band needle blight of Pinus is caused by two distinct species of Dothistroma, D. septosporum and D. pini. Studies in Mycology, (50), 551–565.
Barnes, I., Cortinas, M. N., Wingfield, M. J., & Wingfield, B. D. (2008a). Microsatellite markers for the red band needle blight pathogen, Dothistroma septosporum. Molecular Ecology Resources, 8(5), 1026–1029.
Barnes, I., Kirisits, T., Akulov, A., Chhetri, D. B., Wingfield, B. D., Bulgakov, T. S., & Wingfield, M. J. (2008b). New host and country records of the Dothistroma needle blight pathogens from Europe and Asia. Forest Pathology, 38(3), 178–195.
Barnes, I., Kirisits, T., Wingfield, M. J., & Wingfield, B. D. (2011). Needle blight of pine caused by two species of Dothistroma in Hungary. Forest Pathology, 41(5), 361–369.
Barnes, I., Wingfield, M. J., Carbone, I., Kirisits, T., & Wingfield, B. (2014). Population structure and diversity of an invasive pine needle pathogen reflects anthropogenic activity. Ecology and Evolution, 4(18), 3642–3661.
Bednářová, M., Palovčíková, D., & Jankovský, L. (2006). The host spectrum of Dothistroma needle blight Mycosphaerella pini E. Rostrup - new hosts of Dothistroma needle blight observed in the Czech Republic. Journal of Forest Science, 52(1), 30–36.
Boateng, K., & Lewis, K. J. (2015). Spore dispersal by Dothistroma septosporum in northwest British Columbia. Phytopathology, 105(1), 69–79.
Bradshaw, R. E. (2004). Dothistroma (red-band) needle blight of pines and the dothistromin toxin: a review. Forest Pathology, 34(3), 163–185.
Bradshaw, R. E., Ganley, R. J., Jones, W. T., & Dyer, P. S. (2000). High levels of dothistromin toxin produced by the forest pathogen Dothistroma pini. Mycological Research, 104, 325–332.
Brown, A. V., & Webber, J. (2008). Red band needle blight of conifers in Britain. Research Note - Forestry Commission, (002), 8 pp.
Brown, A. H. D., Feldman, M. W., & Nevo, E. (1980). Multilocus structure of natural populations of Hordeum spontaneum. Genetics, 96(2), 523–536.
Bulman, L., Ganley, R. J., & Dick, M. (2008). Needle diseases of radiata pine in New Zealand. Scion, Client report no. 13010.
Burnett, J. H. (2003). Fungal population genetics and species. Oxford, UK: Oxford University Press.
Burt, A., Carter, D. A., Koenig, G. L., White, T. J., & Taylor, J. W. (1996). Molecular markers reveal cryptic sex in the human pathogen Coccidioides immitis. Proceedings of the National Academy of Sciences, 93(2), 770–773.
Carson, S. D., & Carson, M. J. (1993). Breeding for durable resistance to red band needle blight caused by Dothistroma pini assuming quantitative inheritance. In Durability of disease resistance. (pp. 309–309).
Chen, R.-S., & McDonald, B. A. (1996). Sexual reproduction plays a major role in the genetic structure of populations of the fungus Mycosphaerella graminicola. Genetics, 142(4), 1119–1127.
Dale, A. L., Lewis, K. J., & Murray, B. W. (2011). Sexual reproduction and gene flow in the pine pathogen Dothistroma septosporum in British Columbia. Phytopathology, 101(1), 68–76.
De Villebone, D., & Maugard, F. (1999). Scirrhia pini: un pathogène du feuillage en pleine expansion sur le pin Laricio en France. Bilan de la santé des forêts, 1998, 30–32.
De Wit, P. J. G. M., van der Burgt, A., Ökmen, B., Stergiopoulos, I., Abd-Elsalam, K. A., Aerts, A. L., et al. (2012). The genomes of the fungal plant pathogens Cladosporium fulvum and Dothistroma septosporum reveal adaptation to different hosts and lifestyles but also signatures of common ancestry. PLoS Genetics, 8(11).
Doroguine, G. (1911). Une maladie cryptogamique du pin. Bulletin Trimestriel de la Societe Mycologique de France, 27(1), 105–106.
Drenkhan, R., & Hanso, M. (2009). Recent invasion of foliage fungi of pines (Pinus spp.) to the northern Baltics. Forestry Studies/Metsanduslikud uurimused, 51(1), 49–64.
Drenkhan, R., Hantula, J., Vuorinen, M., Jankovský, L., & Müller, M. M. (2013). Genetic diversity of Dothistroma septosporum in Estonia, Finland and Czech republic. European Journal of Plant Pathology, 136(1), 71–85.
Earl, D. A., & vonHoldt, B. M. (2012). Structure harvester: a website and program for visualizing structure output and implementing the EVANNO method. Conservation Genetics Resources, 4(2), 359–361.
Evanno, G., Regnaut, S., & Goudet, J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology, 14(8), 2611–2620.
Excoffier, L., & Lischer, H. E. L. (2010). ARLEQUIN suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources, 10(3), 564–567.
Fabre, B., Ioos, R., Piou, D., & Marçais, B. (2012). Is the emergence of Dothistroma needle blight of pine in France caused by the cryptic species Dothistroma pini? Phytopathology, 102(1), 47–54.
Falush, D., Stephens, M., & Pritchard, J. K. (2003). Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics, 164(4), 1567–1587.
FAO. (2008). Forest pest species profile: Mycosphaerella pini Rostrup. Food and Agriculture Organization.
Funk, A., & Parker, A. K. (1966). Scirrhia pini n. sp., the perfect state of Dothistroma pini Hulbary. Canadian Journal of Botany, 44(9), 1171–1176.
Ganley, R. J., & Bradshaw, R. E. (2001). Rapid identification of polymorphic microsatellite loci in a forest pathogen, Dothistroma pini, using anchored PCR. Mycological Research, 105(9), 1075–1078.
Gibson, I. A. S. (1972). Dothistroma blight of Pinus radiata. Annual Review of Phytopathology, 10(1), 51–72.
Gibson, I., Christensen, P. S., & Munga, F. M. (1964). First observations in Kenya of a foliage disease of pines caused by Dothistroma pini Hulbary. Commonwealth Forestry Review, 43, 31–48.
Gladieux, P., Zhang, X.-G., Róldan-Ruiz, I., Caffier, V., Leroy, T., Devaux, M., et al. (2010). Evolution of the population structure of Venturia inaequalis, the apple scab fungus, associated with the domestication of its host. Molecular Ecology, 19(4), 658–674.
Groenewald, M., Barnes, I., Bradshaw, R. E., Brown, A. V., Dale, A., Groenewald, J. Z., et al. (2007). Characterization and distribution of mating type genes in the Dothistroma needle blight pathogens. Phytopathology, 97(7), 825–834.
Guichoux, E., Lagache, L., Wagner, S., Chaumeil, P., LéGer, P., Lepais, O., et al. (2011). Current trends in microsatellite genotyping. Molecular Ecology Resources, 11(4), 591–611.
Hirst, P., Richardson, T. E., Carson, S. D., & Bradshaw, R. E. (1999). Dothistroma pini genetic diversity is low in New Zealand. New Zealand Journal of Forestry Science, 29(3), 459–472.
Ioos, R., Fabre, B., Saurat, C., Fourrier, C., Frey, P., & Marçais, B. (2010). Development, comparison, and validation of real-time and conventional PCR tools for the detection of the fungal pathogens causing brown spot and red band needle blights of pine. Phytopathology, 100(1), 105–114.
Jakobsson, M., & Rosenberg, N. A. (2007). CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics, 23(14), 1801–1806.
Jankovský, L., Bednářová, M., & Palovčíková, D. (2004). Dothistroma needle blight Mycosphaerella pini E. Rostrup, a new quarantine pathogen of pines in the CR. Journal of Forest Science, 50(7), 319–326.
McDonald, B. A., & Linde, C. (2002). Pathogen population genetics, evolutionary potential and durable resistance. Annual Review of Phytopathology, 40(1), 349–379.
Milgroom, M. G. (1996). Recombination and the multilocus structure of fungal populations. Annual Review of Phytopathology, 34(1), 457–477.
Morelet, M. (1967). Une maladie des pins, nouvelle pour la France, due à: Scirrhia pini Funk et Parker, et à son stade conidien: Dothistroma pini Hulbary. Bulletin Mensuel de la Société Linnéenne de Lyon, 36, 361–7.
Morelet, M. (1969). Scirrhia pini: Note Complementaire. Bulletin Mensuel de la Société Linnéenne de Lyon, 38, 268–70.
Nei, M. (1987). Molecular evolutionary genetics. New York, USA: Columbia University Press.
Ohm, R. A., Feau, N., Henrissat, B., Schoch, C. L., Horwitz, B. A., Barry, K. W., et al. (2012). Diverse lifestyles and strategies of plant pathogenesis encoded in the genomes of eighteen Dothideomycetes fungi. PLoS Pathogens, 8(12).
Peakall, R., & Smouse, P. E. (2012). GenAlEx 6.5: genetic analysis in excel. Population genetic software for teaching and research—an update. Bioinformatics, 28(19), 2537–2539.
Peterson, G. W. (1973). Infection of Austrian and ponderosa pines by Dothistroma pini in Eastern Nebraska. Phytopathology, 63(8), 1060–1063.
Piškur, B., Hauptman, T., & Jurc, D. (2013). Dothistroma needle blight in Slovenia is caused by two cryptic species: Dothistroma pini and Dothistroma septosporum. Forest Pathology, 43(6), 518–521.
Queloz, V., Wey, T., & Holdenrieder, O. (2014). First record of Dothistroma pini on Pinus nigra in Switzerland. Plant Disease, 98(12), 1744–1744.
Rodas, C. A., Wingfield, M. J., Granados, G. M., & Barnes, I. (2015). Dothistroma Needle Blight: an emerging epidemic caused by Dothistroma septosporum in Colombia. Plant Pathology.
Rosenberg, N. A. (2004). Distruct: a program for the graphical display of population structure. Molecular Ecology Notes, 4(1), 137–138.
Swofford, D. L. (2003). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sunderland: Sinauer Associates.
Szpiech, Z. A., Jakobsson, M., & Rosenberg, N. A. (2008). ADZE: a rarefaction approach for counting alleles private to combinations of populations. Bioinformatics, 24(21), 2498–2504.
Tomšovský, M., Tomešová, V., Palovčíková, D., Kostovčík, M., Rohrer, M., Hanáček, P., & Jankovský, L. (2013). The gene flow and mode of reproduction of Dothistroma septosporum in the Czech Republic. Plant Pathology, 62(1), 59–68.
Watt, M. S., Kriticos, D. J., Alcaraz, S., Brown, A. V., & Leriche, A. (2009). The hosts and potential geographic range of Dothistroma needle blight. Forest Ecology and Management, 257(6), 1505–1519.
Woods, A. J. (2003). Species diversity and forest health in northwest British Columbia. Forestry Chronicle, 79(5), 892–897.
Zhan, J., Pettway, R. E., & McDonald, B. A. (2003). The global genetic structure of the wheat pathogen Mycosphaerella graminicola is characterized by high nuclear diversity, low mitochondrial diversity, regular recombination, and gene flow. Fungal Genetics and Biology, 38(3), 286–297.
Acknowledgments
The authors thank Benoît Marçais for supplying information about survey sites, and Helen Hipperson for help with microsatellite analysis, as well as advice and support from Simon Archer (Imperial College, London). The authors are grateful for the helpful comments of Joan Webber and Kath Tubby which helped improve the manuscript. This study was financially supported by the Forestry Commission, UK and Imperial College London. The authors would like to acknowledge networking support by the COST Action FP1102, DIAROD and the Department of Science and Technology (DST) and ESTAP, South Africa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mullett, M.S., Brown, A.V. & Barnes, I. Population structure and reproductive mode of Dothistroma septosporum in the Brittany peninsula of France. Eur J Plant Pathol 143, 261–275 (2015). https://doi.org/10.1007/s10658-015-0678-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0678-8