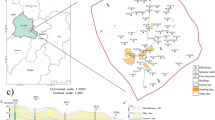
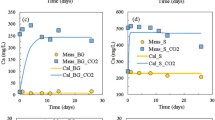
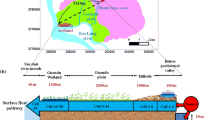
Abstract
Aquifer storage and recovery (ASR) and aquifer recharge (AR) provide technical solutions to address water supply deficits and growing future water demands. Unfortunately, the mobilization of naturally present arsenic due to ASR/AR operations has undermined its application on a larger scale. Predicting arsenic mobility in the subsurface during ASR/AR is further complicated by site-specific factors, including the arsenic mobilization mechanisms, groundwater flow conditions, and multi-phase geochemical interactions. In order to ensure safe and sustainable ASR/AR operation, a better understanding of these factors is needed. The current study thus aims to better characterize and model arsenic remobilization at ASR/AR sites by compiling and analyzing available kinetic data on arsenic mobilization from arsenopyrite under different aqueous conditions. More robust and widely applicable rate laws are developed for geochemical conditions relevant to ASR/AR. Sensitivity analysis of these new rate laws gives further insight into the controlling geochemical factors for arsenic mobilization. When improved rate laws are incorporated as the inputs for reactive transport modeling, arsenic mobilization in ASR/AR operations can be predicted with an improved accuracy. The outcomes will be used to guide groundwater monitoring and specify ASR/AR operational parameters, including water pretreatment requirements prior to injection.





Similar content being viewed by others
References
Asta, M. P., Cama, J., Ayora, C., Acero, P., & de Giudici, G. (2010). Arsenopyrite dissolution rates in O2-bearing solutions. Chemical Geology, 273(3), 272–285.
Baghvand, A., Nasrabadi, T., Bidhendi, G. N., Vosoogh, A., Karbassi, A., & Mehrdadi, N. (2010). Groundwater quality degradation of an aquifer in Iran central desert. Desalination, 260(1), 264–275.
Brenčič, M. (2009). Hydrogeochemistry of coastal carbonate aquifer in Lucija–Portorož (Gulf of Trieste, Northern Adriatic Sea, Slovenia). Acta Carsologica, 38(2–3), 179–196.
Corkhill, C. L., & Vaughan, D. J. (2009). Arsenopyrite oxidation–a review. Applied Geochemistry, 24(12), 2342–2361.
Craw, D., Falconer, D., & Youngson, J. H. (2003). Environmental arsenopyrite stability and dissolution: theory, experiment, and field observations. Chemical Geology, 199(1), 71–82.
Deditius, A. P., Reich, M., Kesler, S. E., Utsunomiya, S., Chryssoulis, S. L., Walshe, J., et al. (2014). The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposits. Geochimica et Cosmochimica Acta, 140, 644–670.
Jones, G. W., & Pichler, T. (2007). Relationship between pyrite stability and arsenic mobility during aquifer storage and recovery in southwest central Florida. Environmental Science and Technology, 41(3), 723–730.
Lazareva, O., Druschel, G., & Pichler, T. (2015). Understanding arsenic behavior in carbonate aquifers: Implications for aquifer storage and recovery (ASR). Applied Geochemistry, 52, 57–66.
McKibben, M. A., Tallant, B. A., & del Angel, J. K. (2008). Kinetics of inorganic arsenopyrite oxidation in acidic aqueous solutions. Applied Geochemistry, 23(2), 121–135.
Mirecki, J. E., Campbell, B. G., Conlon, K. J., & Petkewich, M. D. (1998). Solute changes during aquifer storage recovery testing in a limestone/clastic aquifer. Groundwater, 36(3), 394–403.
Moses, C. O., & Herman, J. S. (1991). Pyrite oxidation at circumneutral pH. Geochimica et Cosmochimica Acta, 55(2), 471–482.
Neil, C. W., & Jun, Y. S. (2015). Fe3 + addition promotes arsenopyrite dissolution and iron (III)(Hydr) oxide formation and phase transformation. Environmental Science & Technology Letters, 3(1), 30–35.
Neil, C. W., Yang, Y. J., & Jun, Y. S. (2012). Arsenic mobilization and attenuation by mineral–water interactions: implications for managed aquifer recharge. Journal of Environmental Monitoring, 14(7), 1772–1788.
Neil, C. W., Yang, Y. J., Schupp, D., & Jun, Y. S. (2014). Water chemistry impacts on arsenic mobilization from arsenopyrite dissolution and secondary mineral precipitation: implications for managed aquifer recharge. Environmental Science and Technology, 48(8), 4395–4405.
Page, D. W., Peeters, L., Vanderzalm, J., Barry, K., & Gonzalez, D. (2017). Effect of aquifer storage and recovery (ASR) on recovered stormwater quality variability. Water Research, 117, 1–8.
Paktunc, D. (2008). Speciation of arsenic in pyrite by micro-X-ray absorption fine-structure spectroscopy (XAFS). Advanced Photon Source: Advanced Photon Source (APS), Argonne National Laboratory (ANL).
Reich, M., & Becker, U. (2006). First-principles calculations of the thermodynamic mixing properties of arsenic incorporation into pyrite and marcasite. Chemical Geology, 225(3), 278–290.
Rosenman, K. D. (2011). Cardiovascular disorders. In B. Levy, D. Wegman, S. Baron, & R. Sokas (Eds.), Occupational and environmental health: Recognizing and preventig disease and injury (pp. 492–504). New York: Oxford University Press.
Sacks, L. A., & Tihansky, A. B. (1996). Geochemical and isotopic composition of ground water, with emphasis on sources of sulfate, in the Upper Floridan Aquifer and Intermediate Aquifer System in Southwest Florida. US Geological Survey: US Department of the Interior.
Schlieker, M., Schüring, J., Hencke, J., & Schulz, H. D. (2001). The influence of redox processes on trace element mobility in a sandy aquifer—An experimental approach. Journal of Geochemical Exploration, 73(3), 167–179.
Seibert, S., Descourvieres, C., Skrzypek, G., Deng, H., & Prommer, H. (2017). Model-based analysis of δ34S signatures to trace sedimentary pyrite oxidation during managed aquifer recharge in a heterogeneous aquifer. Journal of Hydrology, 548, 368–381.
Shiklomanov, I. A., & Rodda, J. C. (Eds.). (2004). World water resources at the beginning of the twenty-first century. Cambridge: Cambridge University Press.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17(5), 517–568.
Smith, W. B., Miller, G. R., & Sheng, Z. (2017). Assessing aquifer storage and recovery feasibility in the Gulf Coastal Plains of Texas. Journal of Hydrology: Regional Studies, 14, 92–108.
Vacher, H. L., Hutchings, W. C., & Budd, D. A. (2006). Metaphors and models: The ASR bubble in the Floridan aquifer. Groundwater, 44(2), 144–154.
Vanderzalm, J. L., Dillon, P. J., Barry, K. E., Miotlinski, K., Kirby, J. K., & La Salle, C. L. G. (2011). Arsenic mobility and impact on recovered water quality during aquifer storage and recovery using reclaimed water in a carbonate aquifer. Applied Geochemistry, 26(12), 1946–1955.
Walker, F. P., Schreiber, M. E., & Rimstidt, J. D. (2006). Kinetics of arsenopyrite oxidative dissolution by oxygen. Geochimica et Cosmochimica Acta, 70(7), 1668–1676.
Wallis, I., Prommer, H., Pichler, T., Post, V. B., Norton, S., Annable, M. D., et al. (2011). Process-based reactive transport model to quantify arsenic mobility during aquifer storage and recovery of potable water. Environmental Science and Technology, 45(16), 6924–6931.
Welch, A., Ryker, S., Helsel, D., & Hamilton, P. (2001). Arsenic in Ground Water of the United States: An overview. Water Well Journal, February 2001, 30–33.
Yaron, B., Dror, I., & Berkowitz, B. (2012). Soil-subsurface change: Chemical pollutant impacts. Berlin: Springer.
Yu, Y., Zhu, Y., Gao, Z., Gammons, C. H., & Li, D. (2007). Rates of arsenopyrite oxidation by oxygen and Fe(III) at pH 1.8– 12.6 and 15–45 C. Environmental Science and Technology, 41(18), 6460–6464.
Yuan, J., Van Dyke, M. I., & Huck, P. M. (2017). Identification of critical contaminants in wastewater effluent for managed aquifer recharge. Chemosphere, 172, 294–301.
Yunmei, Y., Yongxuan, Z., Williams-Jones, A. E., Zhenmin, G., & Dexian, L. (2004). A kinetic study of the oxidation of arsenopyrite in acidic solutions: implications for the environment. Applied Geochemistry, 19(3), 435–444.
Zektser, S., Loáiciga, H. A., & Wolf, J. T. (2005). Environmental impacts of groundwater overdraft: selected case studies in the southwestern United States. Environmental Geology, 47(3), 396–404.
Acknowledgements
The research effort in this paper is a part of the EPA’s Safe and Sustainable Water Resources (SSWR) and Air Climate and Energy (ACE) research program. Funding and resources from these programs are acknowledged. CWN acknowledges the generous support of the Oak Ridge Institute for Science and Education (ORISE) program. Furthermore, we thank two anonymous reviewers for their constructive comments. This paper has been subjected to the Agency’s administrative review and has been approved for external publication. Any opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Agency or ORISE; therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Neil, C.W., Jason Todd, M. & Jeffrey Yang, Y. Improving arsenopyrite oxidation rate laws: implications for arsenic mobilization during aquifer storage and recovery (ASR). Environ Geochem Health 40, 2453–2464 (2018). https://doi.org/10.1007/s10653-018-0111-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-018-0111-2