Summary
Background
Part E of the KEYNOTE-011 (NCT01840579) study assessed the safety and antitumor activity of pembrolizumab plus platinum-etoposide chemotherapy in Japanese patients with previously untreated extensive-stage small-cell lung cancer (ES-SCLC).
Methods
Patients received 4 cycles of pembrolizumab (200 mg) every 3 weeks in combination with cisplatin (75 mg/m2) and etoposide (100 mg/m2; days 1, 2, 3) in cohort 1; with carboplatin (AUC 5 mg/mL/min) and etoposide (100 mg/m2; days 1, 2, 3) in cohort 2; or with cisplatin/etoposide and pegfilgrastim (3.6 mg; cycle 1, day 4) in cohort 3. Combination therapy was followed by pembrolizumab monotherapy (31 cycles). The primary endpoint was safety and tolerability (including dose-limiting toxicities; DLTs).
Results
Fifteen patients were included in the study (cohort 1, n = 6; cohort 2, n = 6; cohort 3, n = 3). Median time from treatment allocation to data cutoff was 22.1 months (range, 4.1‒32.4 months). DLTs occurred in 3 patients in cohort 1 (one patient with grade 4 laryngeal stenosis and grade 3 febrile neutropenia; two patients with grade 3 febrile neutropenia); no patients in cohorts 2 or 3 experienced DLTs. Grade ≥ 3 treatment-related adverse events included leukopenia (67%) and neutropenia (87%). Among all patients, ORR was 67% (95% CI, 38%‒88%) and median DOR was 4.5 months (range, 2.8‒28.8 months). Median PFS was 4.2 months (95% CI, 3.0‒7.8 months) and median OS was 22.1 months (95% CI, 7.4‒25.9 months).
Conclusion
Pembrolizumab in combination with platinum-etoposide therapy had manageable toxicity with no new safety signals and was associated with antitumor activity in Japanese patients with ES-SCLC.
Trial Registration
ClinicalTrials.gov, NCT01840579.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer death in Japan; in 2020, it was estimated to account for 13% of new cancer diagnoses and 20% of cancer deaths [1]. Based on a registry study of patients with lung cancer receiving medical treatment, small-cell lung cancer (SCLC) accounts for 19% of all lung cancers in Japan [2]. Until recently, first-line treatment for patients with extensive-stage (ES) SCLC was typically platinum-based chemotherapy with etoposide [3]. Despite such treatments, outcomes for patients with SCLC have been very poor: median overall survival (OS) is approximately 7 months, and the 5-year survival rate is approximately 6% [4].
The programmed cell death protein 1 (PD-1) pathway is frequently altered in cancer, leading to inhibition of active T-cell‒mediated immune surveillance of tumors. Consequently, PD-1 and its ligands (programmed death ligand 1 and 2 [PD-L1 and PD-L2]) have been the targets of anticancer immunotherapies [5,6,7]. SCLC is associated with heavy smoking and a high mutation burden [8], suggesting that such tumors may be immunogenic and that immunotherapies may be effective in the treatment of SCLC. Immunotherapies that block PD-1 pathway signaling have been shown to improve outcomes in patients with ES-SCLC. In the IMpower133 trial, adding the anti‒PD-L1 monoclonal antibody atezolizumab to platinum-etoposide chemotherapy resulted in improved OS (hazard ratio [HR], 0.70; 95% CI, 0.54‒0.91; P = 0.007) and progression-free survival (PFS; HR, 0.77; 95% CI, 0.62‒0.96; P = 0.02) compared with placebo plus platinum-etoposide [9]. In the CASPIAN trial, hazard ratios for OS (0.73; 95% CI, 0.59–0.91; P = 0.0047) and PFS (0.78; 95% CI, 0.65–0.94; statistical significance not evaluated) both favored treatment with the anti‒PD-L1 monoclonal antibody durvalumab plus platinum-etoposide compared with placebo plus platinum-etoposide [10].
Pembrolizumab, a humanized anti‒PD-1 monoclonal antibody, blocks the interaction between PD-1 and its ligands PD-L1 and PD-L2. Pembrolizumab monotherapy showed antitumor activity in patients with previously treated ES-SCLC in the KEYNOTE-028 and KEYNOTE-158 studies [11,12,13]. In a pooled analysis of patients from KEYNOTE-028 and KEYNOTE-158, the objective response rate (ORR) was 19% [13]. Addition of pembrolizumab to platinum-etoposide chemotherapy in the phase 3 KEYNOTE-604 study significantly prolonged PFS compared with placebo plus chemotherapy (HR, 0.75; 95% CI, 0.61‒0.91; P = 0.0023); however, OS was not significantly improved (HR, 0.80; 95% CI, 0.64‒0.98; P = 0.0164) [14].
KEYNOTE-011 (NCT01840579) is an open-label, multipart, phase 1 study of pembrolizumab in a Japanese population of patients with advanced solid tumors. We report results from part E, which assessed the safety and antitumor activity of pembrolizumab in combination with standard platinum-etoposide chemotherapy in Japanese patients with ES-SCLC.
Methods
Patients
Patients were eligible for inclusion in part E of the KEYNOTE-011 study if they were ≥ 20 years of age with previously untreated, histologically or cytologically confirmed ES-SCLC (stage IV or T3‒4 due to presence of multiple lung nodules per the American Joint Committee of Cancer, 7th edition), an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and ≥ 1 radiographically measurable lesion per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (defined as a lesion ≥ 10 mm in the longest diameter or lymph node ≥ 15 mm in the short axis). Patients were required to have adequate organ function, including hematologic, renal, hepatic, endocrine, and coagulation laboratory values. Patients were excluded from the study if they had radiation therapy, chronic systemic steroid therapy, or any other immunosuppressive therapy ≤ 2 weeks before the first dose of study treatment. Also excluded were patients who had not recovered from adverse events (AEs) due to previous treatment > 4 weeks prior to the first dose of study treatment; had a history of acute diverticulitis, intra-abdominal abscess, gastrointestinal obstruction, or abdominal carcinomatosis; had a history of hematologic malignancy, primary brain tumor or sarcoma, or another primary solid tumor unless they had received potentially curative therapy with no evidence of disease for ≥ 5 years; had known active central nervous system metastases and/or carcinomatous meningitis (patients could participate in the study if they were clinically stable for at least 2 weeks with no evidence of new or enlarging brain metastases and were off steroids 3 days before dosing with study medication); had prior therapy with an anti‒PD-1, anti‒PD-L1, or anti‒PD-L2 agent or an antibody targeting other immune-regulatory receptors or mechanisms; or had participated in a study of an investigational agent or device within 30 days of study treatment.
Study design
Patients received up to 4 cycles of intravenous pembrolizumab (200 mg on day 1 of each cycle) every 3 weeks (Q3W) in combination with intravenous cisplatin (75 mg/m2 on day 1) and etoposide (100 mg/m2 on days 1, 2, and 3) in cohort 1; with intravenous carboplatin (area under the curve 5 mg/mL/min on day 1) and etoposide (100 mg/m2 on days 1, 2, and 3) in cohort 2; or with intravenous cisplatin (75 mg/m2 on day 1) and etoposide (100 mg/m2 on days 1, 2, and 3) with prophylactic subcutaneous pegylated granulocyte colony-stimulating factor (G-CSF; pegfilgrastim; 3.6 mg on day 4 of cycle 1) in cohort 3. Combination therapy was followed by pembrolizumab monotherapy (200 mg Q3W for up to 31 cycles). Treatment was discontinued upon completion of 2 years of study treatment, documented disease progression, or unacceptable AEs.
Endpoints
The primary endpoint was safety and tolerability of pembrolizumab, including dose-limiting toxicities (DLTs), AEs, and laboratory tests. DLTs were evaluated at completion of the DLT evaluation period in each patient (first 3 weeks after initiation of study treatments). The following events were considered DLTs if assessed as related to treatment by the investigator: grade 4 neutropenia lasting > 7 days, grade 3 or 4 febrile neutropenia (absolute neutrophil count < 1000/mm3 with single temperature > 38.3 °C or a sustained temperature ≥ 38 °C for > 1 h; for grade 4, life-threatening consequences and urgent intervention indicated), grade 4 thrombocytopenia (< 25,000/mm3), grade 4 anemia, grade 4 non-hematologic toxicity, grade 3 non-hematologic toxicity lasting > 3 days despite optimal supportive care, and any grade 3 non-hematologic laboratory values if medical intervention was required to treat the patient or the abnormality persisted for > 7 days. The target DLT rate was 30% using a toxicity profile interval design [15]. Exploratory endpoints included ORR, defined as the proportion of patients with a complete response (CR) or partial response (PR); duration of response (DOR), defined as the time from first evidence of CR or PR to disease progression or death; PFS, defined as the time from treatment allocation to the first of disease progression or death; and OS, defined as the time from treatment allocation to death.
Assessments
All toxicities were graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. AEs were reported from the time of treatment allocation through 30 days following cessation of treatment; serious AEs were collected for up to 90 days after cessation of treatment or 30 days after cessation of treatment if the patient initiated new anticancer therapy, whichever was earlier.
Tumor imaging by computed tomography (CT) or magnetic resonance imaging (MRI) was done at baseline, every 6 weeks for the first 24 weeks, then every 9 weeks thereafter. Antitumor activity was evaluated based on CT or MRI per RECIST version 1.1 by investigator assessment. Patients who experienced progressive disease (PD) or started another treatment were followed for survival every 2 months from the last contact in the study.
Statistics
Between 3 and 9 patients were planned to be enrolled in each cohort. Patients who received ≥ 1 dose of study treatment were included in the analysis. Data were summarized using descriptive statistics. DLT-evaluable patients had a DLT in the DLT evaluation period or had received ≥ 90% of the prescribed dose of pembrolizumab and the combination regimen and completed all safety evaluations in the DLT evaluation period without experiencing a DLT. DOR, PFS, and OS were summarized using the Kaplan-Meier method.
Results
A total of 15 patients were enrolled in part E of the KEYNOTE-011 study between March 10, 2017, and February 28, 2020 (data cutoff): 6 patients in cohort 1 (pembrolizumab plus cisplatin and etoposide), 6 patients in cohort 2 (pembrolizumab plus carboplatin and etoposide), and 3 patients in cohort 3 (pembrolizumab plus cisplatin, etoposide, and prophylactic pegfilgrastim). Database lock was June 11, 2020.
Patient characteristics are summarized in Table 1. Median age was 63.5 years in cohort 1, 65.0 years in cohort 2, and 57.0 years in cohort 3. Overall, median age was 64 years, and approximately half of patients included in the study were men. Three patients in cohort 1, 1 patient in cohort 2, and 1 patient in cohort 3 had brain metastases at baseline.
Median time from treatment allocation to data cutoff (February 28, 2020) was 16.1 months (range, 4.1‒31.4 months) for cohort 1, 17.7 months (range, 6.4‒32.4 months) for cohort 2, and 22.1 months (range, 8.9‒25.6 months) for cohort 3. Across all 3 cohorts, median time from treatment allocation to data cut off was 22.1 months (range, 4.1‒32.4 months). All patients discontinued study treatment due to PD (n = 12), AEs (n = 2), or physician decision (n = 1; Fig. 1). Median treatment exposure was 2.8 months (range, 0.1‒13.4 months) in cohort 1, 4.5 months (range, 2.2‒7.7 months) in cohort 2, and 3.5 months (range, 3.5‒4.9 months) in cohort 3. Median number of pembrolizumab administrations was 5 (range, 1‒19) in cohort 1, 7 (range, 3‒11) in cohort 2, and 6 (range, 5‒8) in cohort 3. Only 3 patients remained in follow-up when the study was terminated by the sponsor because evaluation of the primary objective had been completed. Overall, 13 of 15 patients received subsequent anticancer therapy after discontinuing study treatment (Online Resource 1).
Safety
Three patients in cohort 1 experienced a DLT of grade 3 febrile neutropenia during cycle 1. One of these patients also experienced grade 4 laryngeal stenosis that was considered a DLT and discontinued from the study. The other 2 patients received filgrastim and meropenem or cefepime and were able to continue to cycle 2 without delay; the etoposide dose was reduced to 80 mg/m2 for both patients, and the cisplatin dose was reduced to 60 mg/m2 for 1 patient. No patients in cohort 2 or cohort 3 experienced a DLT.
All 15 patients receiving pembrolizumab in combination with platinum-etoposide chemotherapy experienced ≥ 1 treatment-related AE; the incidence of AEs was similar across all 3 cohorts (Table 2). Discontinuation due to treatment-related AEs was reported in 2 patients (1 each in cohort 1 and cohort 2). No AEs led to death. Serious treatment-related AEs were interstitial lung disease, febrile neutropenia, and laryngeal stenosis (n = 1 each) in cohort 1 and peripheral sensory neuropathy and lichenoid keratosis (n = 1 each) in cohort 2; no serious AEs were reported in cohort 3. Across all cohorts, the most frequently occurring treatment-related AEs included leukopenia, neutropenia, and anemia (Table 2). The most frequently occurring grade 3 or 4 treatment-related AEs were neutropenia, leukopenia, and febrile neutropenia (Table 2).
Immune-mediated AEs were pneumonitis (n = 2) in cohort 1 and adrenal insufficiency, hyperthyroidism, and hypothyroidism (n = 1 each) in cohort 3 (Table 2). The only grade ≥ 3 immune-mediated AE was pneumonitis reported in cohort 1 (Table 2). No grade ≥ 3 immune-mediated AEs were reported in cohort 2 or cohort 3.
Antitumor activity
The ORR across all patients was 67% (95% CI, 38%‒88%). All 10 responses were PRs; no patient experienced a CR (Fig. 2A). The ORR was 50% (95% CI, 12%‒88%) in cohort 1, 83% (95% CI, 36%‒100%) in cohort 2, and 67% (95% CI, 9%‒99%) in cohort 3. Median time to response in all patients was 1.4 months (range, 1.2‒3.0 months) and median DOR was 4.5 months (range, 2.8‒28.8 months). Reductions in tumor size from baseline occurred in 14 of 15 patients (n = 8 in cohorts 1 and 3 combined and n = 6 in cohort 2; Fig. 2B, C).
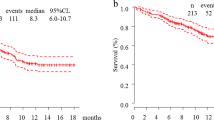
Survival and duration of response in individual patients (A), best change in sum of diameters of target lesions from baseline in individual patients (B), and percentage change in diameters of target lesions over time in individual patients (C). In panel A, bar lengths indicate follow-up duration. PD, progressive disease; PR, partial response; SD, stable disease
At the time of data cutoff, 14 of 15 patients (93%) had experienced a PFS event. Across all patients, median PFS was 4.2 months (95% CI, 3.0‒7.8 months), with an estimated PFS rate of 80% (95% CI, 50.0%‒93.1%) at 3 months and 40% (95% CI, 16.5%‒62.8%) at 6 months. Median PFS was 4.8 months (95% CI, 1.6 months‒not reached) for patients in cohort 1, 7.7 months (95% CI, 3.0‒30.2 months) for patients in cohort 2, and 4.2 months (95% CI, 3.9‒4.2 months) for patients in cohort 3.
Twelve of the 15 patients had died by the data cutoff date. Median OS among all patients was 22.1 months (95% CI, 7.4‒25.9 months), with an estimated 12-month survival rate of 53%. Median OS was 16.1 months (95% CI, 3.7 months‒not reached) in patients in cohort 1, 17.7 months (95% CI, 6.4 months‒not reached) in cohort 2, and 22.1 months (95% CI, 8.3 months‒not reached) in cohort 3.
Discussion
Pembrolizumab in combination with platinum-etoposide chemotherapy was generally well tolerated and showed antitumor activity in Japanese patients with ES-SCLC. DLT rates were 20% (3/15); all patients with a DLT received pembrolizumab in combination with cisplatin and etoposide (ie, cohort 1). Furthermore, this AE profile was generally consistent with findings in a global population receiving pembrolizumab and etoposide combined with either carboplatin or cisplatin for SCLC [14]. As anticipated based on previous reports evaluating pembrolizumab as monotherapy in patients with recurrent or metastatic SCLC [13] or in combination with etoposide-platinum in patients with ES-SCLC [14], previous reports evaluating pembrolizumab, and the immune-mediated mechanism of action for pembrolizumab, immune-mediated AEs were observed, including pneumonitis, adrenal insufficiency, hyperthyroidism, and hypothyroidism. One event of grade 3 pneumonitis occurred in 1 patient in cohort 1; no other grade ≥ 3 immune-mediated AEs were observed.
In this study, the ORR among all patients was 67% (95% CI, 38%‒88%), with a median time to response of 1.4 months (range, 1.2‒3.0 months) and median DOR of 4.5 months (range, 2.8‒28.8 months). While comparison with other studies may be difficult because of the small sample size, these results were consistent with the KEYNOTE-604 study, in which the ORR was 71% (95% CI, 64%‒76%) in the pembrolizumab plus etoposide and platinum group [14]. Among the 15 patients included in the current analysis, nearly all experienced a reduction in tumor size from baseline. Median PFS was 4.2 months (95% CI, 3.0‒7.8 months) and median OS was 22.1 months (95% CI, 7.4‒25.9 months). In the global population enrolled in KEYNOTE-604, pembrolizumab in combination with etoposide and platinum resulted in a similar median PFS of 4.5 months (95% CI, 4.3‒5.4 months) and DOR of 4.2 months (range, 1.0+ to 26.0+ months). However, median OS was 10.8 months (95% CI, 9.2‒12.9 months) in KEYNOTE-604 [14] and thus was markedly shorter than the median OS of 22.1 months (95% CI, 7.4‒25.9 months) in KEYNOTE-011 part E. Results from KEYNOTE-011 part E support the hypothesis that the combination of immune checkpoint inhibitors with chemotherapy provides antitumor activity and can improve outcomes in patients with SCLC.
Limitations of the study include the small number of patients and open-label design. Despite these limitations, the results of this study suggest that the effects of pembrolizumab in a Japanese population are consistent with previous reports in global clinical studies of patients with ES-SCLC.
KEYNOTE-011 part E found that pembrolizumab in combination with platinum-etoposide therapy had manageable toxicity and demonstrated antitumor activity in a Japanese population with ES-SCLC. The current study provides encouraging evidence for immunotherapy targeting the PD-1 pathway in Japanese patients with ES-SCLC. Ongoing studies are evaluating pembrolizumab in combination with chemotherapy and other agents, such as olaparib in patients with limited-stage SCLC (NCT04624204) and as a coformulation with the anti‒T-cell immunoglobulin and ITIM domain inhibitor vibostolimab (TIGIT; NCT05224141), in patients with ES-SCLC.
Data sharing
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
References
Foundation for Promotion of Cancer Research (2021) Cancer statistics in Japan - 2021. https://ganjoho.jp/public/qa_links/report/statistics/pdf/cancer_statistics_2021.pdf. Accessed 22 Nov 2021
Sekine I, Shintani Y, Shukuya T, Takayama K, Inoue A, Okamoto I, Kiura K, Takahashi K, Dosaka-Akita H et al (2020) A Japanese lung cancer registry study on demographics and treatment modalities in medically treated patients. Cancer Sci 111:1685–1691. https://doi.org/10.1111/cas.14368
Rudin CM, Ismaila N, Hann CL, Malhotra N, Movsas B, Norris K, Pietanza MC, Ramalingam SS, Turrisi AT 3rd et al (2015) Treatment of small-cell lung cancer: American Society of Clinical Oncology endorsement of the American College of Chest Physicians guideline. J Clin Oncol 33:4106–4111. https://doi.org/10.1200/JCO.2015.63.7918
Wang S, Tang J, Sun T, Zheng X, Li J, Sun H, Zhou X, Zhou C, Zhang H et al (2017) Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep 7:1339. https://doi.org/10.1038/s41598-017-01571-0
Zitvogel L, Kroemer G (2012) Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 1:1223–1225. https://doi.org/10.4161/onci.21335
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563–567. https://doi.org/10.1038/nature14011
Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E, Watkins A, Mullins S, Chodorge M et al (2015) Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res 3:1052–1062. https://doi.org/10.1158/2326-6066.CIR-14-0191
George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G, Leenders F, Lu X, Fernandez-Cuesta L et al (2015) Comprehensive genomic profiles of small cell lung cancer. Nature 524:47–53. https://doi.org/10.1038/nature14664
Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML et al (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379:2220–2229. https://doi.org/10.1056/NEJMoa1809064
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Ozguroglu M et al (2019) Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394:1929–1939. https://doi.org/10.1016/s0140-6736(19)32222-6
Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, Piperdi B, Mehnert JM (2017) Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol 35:3823–3829. https://doi.org/10.1200/JCO.2017.72.5069
Chung HC, Lopez-Martin JA, Kao SC-H, Miller WH, Ros W, Gao B, Marabelle A, Gottfried M, Zer A et al (2018) Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J Clin Oncol 36:8506–8506. https://doi.org/10.1200/JCO.2018.36.15_suppl.8506
Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens JHM, Kao S, Miller WH Jr, Delord JP, Gao B, Planchard D et al (2020) Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol 15:618–627. https://doi.org/10.1016/j.jtho.2019.12.109
Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, Cheema PK, Rodriguez-Abreu D, Wollner M et al (2020) Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol 38:2369–2379. https://doi.org/10.1200/jco.20.00793
Ji Y, Li Y, Nebiyou Bekele B (2007) Dose-finding in phase I clinical trials based on toxicity probability intervals. Clin Trials 4:235–244. https://doi.org/10.1177/1740774507079442
Acknowledgements
We thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. Project management was provided by Masaru Watanabe, PhD, of MSD K.K. (Tokyo, Japan). Medical writing assistance was provided by Natalia Zhukovskaya, PhD, of ICON plc (Blue Bell, PA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Funding
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
Takashi Seto and Yoshitaka Zenke contributed towards the conception, design, or planning of the study. Miyako Satouchi and Takashi Seto provided study materials/patients. Naoyuki Nogami and Yoshitaka Zenke contributed towards the drafting of the manuscript. All authors contributed to the acquisition, analysis, or interpretation of the data, reviewed the manuscript for important intellectual content, and agreed to submit the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by an institutional review board or independent ethics committee at each study site. The trial was conducted in accordance with Good Clinical Practice guidelines and in compliance with the Declaration of Helsinki. Patients provided informed consent.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Disclosures
Dr. Nogami reports receiving lecture fees, honoraria, or other fees from AstraZeneca, Chugai Pharmaceutical Co., Ltd., and Eli Lilly Japan. Takaaki Tokito has received honoraria for lectures from Chugai Pharmaceutical, AstraZeneca, MSD, and Novartis. Yoshitaka Zenke has received payment or honoraria from AstraZeneca, Eli Lilly, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, Takeda Pharmaceutical, Boehringer-Ingelheim, Taiho, MSD, Novartis, Pfizer, Nippon-Kayaku, and Kyowa-Hakko Kirin; institution has received grants or contracts from MSD, Merck, AstraZeneca, Daiichi-Sankyo, and Amgen. Miyako Satouchi has received honoraria (lecture fees) from Chugai, AstraZeneca, Eli Lilly, Taiho, MSD, Pfizer, Novartis, Bristol-Myers Squibb, Ono, Takeda, Daiichi-Sankyo, Amgen, and Merck; and has received clinical trial funding from Chugai, AstraZeneca, MSD, Janssen, Amgen, Taiho, Bristol-Myers Squibb, Pfizer, Daiichi-Sankyo, and Eisai. Takashi Seto has received honoraria for lectures from AstraZeneca, Bristol-Myers Squibb, Chugai Pharmaceutical, Covidien Japan, Daiichi Sankyo, Eli Lilly Japan, Kyowa Hakko Kirin, MSD, Mochida Pharmaceutical, Nippon Boehringer Ingelheim, Novartis Pharma, Ono Pharmaceutical, Pfizer Japan, Taiho Pharmaceutical, Takeda Pharmaceutical, and Towa Pharmaceutical; institution has received grants or contracts from AbbVie, Chugai Pharmaceutical, Daiichi Sankyo, Eli Lilly Japan, Kissei Pharmaceutical, MSD, Novartis Pharma, and Pfizer Japan. Hideo Saka has received honoraria from Boston Scientific, Taiho, AstraZeneca, Ono, Boehringer-Ingelheim, Fuji film, JSRE, Eli Lilly, Chugai, Kaneka, and Century-Medical; has received research funding from Boston Scientific, MSD, AstraZeneca, Ono, Parexel International, WJOG, Celgene, and Taisho-Toyama. Junko Ohtani, Shirong Han, and Kazuo Noguchi are employees of MSD K.K., Tokyo, Japan. Makoto Nishio has received grants and personal fees from Ono Pharmaceutical, Bristol Myers Squibb, Pfizer, Chugai Pharmaceuticals, Eli Lilly, Taiho Pharmaceutical, AstraZeneca, MSD, Novartis, Daiichi Sankyo, and Takeda Pharmaceutical Company Limited; and has received personal fees from Boehringer-Ingelheim, Merck Biopharma, Teijin Pharma Limited, and AbbVie.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nogami, N., Tokito, T., Zenke, Y. et al. Phase 1 study of pembrolizumab plus chemotherapy in Japanese patients with extensive-stage small-cell lung cancer. Invest New Drugs 42, 136–144 (2024). https://doi.org/10.1007/s10637-023-01411-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-023-01411-1