Summary
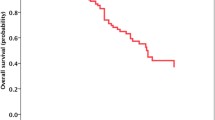
Objectives: The study was designed to investigate the safety of ramucirumab administered in combination with erlotinib or osimertinib for patients with untreated EGFR-mutated non–small cell lung cancer (NSCLC) and asymptomatic brain metastases, a patient subgroup in which these regimens have remained untested. Materials and methods: This phase 1b study (RELAY-Brain) consisted of two cohorts with three patients each. Patients with asymptomatic brain metastases received ramucirumab every 2 weeks plus either daily oral erlotinib or osimertinib until disease progression or intolerable toxicity. The primary objective was to assess dose-limiting toxicity (DLT), defined as central nervous system (CNS) hemorrhage of grade ≥ 2. Results: Six patients were enrolled. Neither DLT nor serious or unexpected adverse events were observed. One treatment-related adverse event of grade ≥ 3 (hypertension of grade 3) was apparent. Common adverse events were generally manageable. The median number of ramucirumab administrations was 18.5 (range, 13 to 31), and there were no detected episodes of CNS hemorrhage. Five of the six patients showed an objective systemic response. Although only one patient had a measurable CNS lesion at baseline, a confirmed intracranial partial response was observed. Conclusion: Ramucirumab in combination with erlotinib or osimertinib showed safety for EGFR-mutated NSCLC with brain metastases.

Similar content being viewed by others
Availability of data and materials
Data are available.
References
Maemondo M et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362(25):2380–2388
Soria JC et al (2018) Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 378(2):113–125
Saito H et al (2019) Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 20(5):625–635
Nakagawa K et al (2019) Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20(12):1655–1669
Besse B et al (2015) Bevacizumab in Patients with Nonsquamous Non-Small Cell Lung Cancer and Asymptomatic, Untreated Brain Metastases (BRAIN): A Nonrandomized Phase II Study. Clin Cancer Res 21(8):1896–1903
Socinski MA et al (2009) Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol 27(31):5255–5261
DiLuna ML et al (2007) Prognostic factors for survival after stereotactic radiosurgery vary with the number of cerebral metastases. Cancer 109(1):135–145
Sekine A et al (2012) Metastatic brain tumors from non-small cell lung cancer with EGFR mutations: distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer 77(1):64–69
Passaro A et al (2019) Brain metastases in EGFR-positive non-small cell lung cancer: the way to the sanctuary becomes less winding. Ann Transl Med 7(Suppl 3):S80
Ilhan-Mutlu A et al (2016) Bevacizumab Prevents Brain Metastases Formation in Lung Adenocarcinoma. Mol Cancer Ther 15(4):702–710
Masuda C et al (2020) Bevacizumab suppresses the growth of established non-small-cell lung cancer brain metastases in a hematogenous brain metastasis model. Clin Exp Metastasis 37(1):199–207
Chikaishi Y et al (2018) Effect of erlotinib plus bevacizumab on brain metastases in patients with non-small cell lung cancer. Ann Transl Med 6(20):401
Feng PH et al (2018) Bevacizumab Reduces S100A9-Positive MDSCs Linked to Intracranial Control in Patients with EGFR-Mutant Lung Adenocarcinoma. J Thorac Oncol 13(7):958–967
Acknowledgements
This study was conducted with support from both the Translational Research Center for Medical Innovation, Foundation for Biomedical Research and Innovation at Kobe, Japan, and the Center for Clinical Research and Innovation, Osaka City University hospital, Osaka, Japan. We thank Chikako Minami and Mieko Nishiu as members of the study support staff.
Funding
This work was supported by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
H.K. and K.S. participated in the conception and design of this study. H.K had a funding acquisition. H.K drafted the article. All authors critically reviewed and contributed to all drafts, approved the final version, and made the decision to submit the report for publication.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the institutional review board of each participating institution and was conducted in compliance with the ethical principles of the Declaration of Helsinki, the International Council for Harmonisation Good Clinical Practice Guideline, and applicable local regulations.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
Dr. Kaneda reports grants from Eli Lilly, during the conduct of the study; personal fees from Chugai, Astrazeneca, Novartis Pharma, Bristol-Myers Squibb, Ono, Boehringer Ingelheim, MSD, Taiho, Pfizer, Nippon KAYAKU, and Merck, outside the submitted work. Dr. Sawa reports personal fees from Chugai Pharmaceutical, Daiichi Sankyo, and Nippon Boehringer Ingelheim, all outside the submitted work. Dr. Daga reports personal fees from Chugai, and Ono; grants from Astrazeneca, all outside the submitted work. Dr. Atagi reports grants and personal fees from AstraZeneca, Ono, Taiho Pharmaceutical, Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb, MSD, Eli Lilly, Chugai, and Merck; grants and non-financial support from F. Hoffmann-La Roche; personal fees from Hisamitsu, Kyowa Hakko Kirin, Novartis Pharma, and Thermo Fisher Scientific, all outside the submitted work. Dr. Mitsuoka reports personal fees from Ono, Bristol-Myers Squibb, and Kyowa Hakko Kirin; grants from Taiho Pharmaceutical, all outside the submitted work. Dr. Kawaguchi reports grants and personal fees from Chugai Pharma, Taiho Pharmaceutical, Boehringer Ingelheim, Ono Pharmaceutical, and Bristol-Myers Squibb Japan; grants, personal fees and non-financial support from Eli Lilly; personal fees from Astra Zeneca, MSD Oncology, Pfizer, Novartis, and Astellas Pharma; grants from Daiichi Sankyo,all outside the submitted work. All remaining authors have declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaneda, H., Sawa, K., Daga, H. et al. Phase 1b study of ramucirumab in combination with erlotinib or osimertinib for untreated EGFR-mutated non–small cell lung cancer patients with asymptomatic brain metastases. Invest New Drugs 39, 1598–1603 (2021). https://doi.org/10.1007/s10637-021-01147-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01147-w