Summary
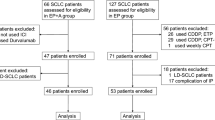
Although immune checkpoint inhibitors have improved the survival of small cell lung cancer (SCLC) patients, their efficacy in SCLC patients who relapsed after systemic chemotherapy is unclear. This retrospective study aimed to investigate the utility of treatment with atezolizumab plus carboplatin and etoposide in SCLC patients previously treated with platinum-based chemotherapy. We retrospectively screened consecutive eight SCLC patients who received atezolizumab plus carboplatin and etoposide after platinum-based chemotherapy. We evaluated the efficacy of this treatment and its association with programmed cell death-ligand 1 (PD-L1) expression. Three and five patients had sensitive relapse and refractory relapse for first-line platinum-based chemotherapy, respectively. The overall response rate and disease control rate was 37.5% and 75.0%, respectively. Median progression-free survival was 4.0 months. Out of three patients who achieved clinical response, two patients had refractory relapse for first-line platinum-based chemotherapy. No patient exhibited PD-L1 expression. Atezolizumab plus carboplatin and etoposide therapy was effective in SCLC patients with sensitive and refractory relapse and might be a second-line treatment option for SCLC patients previously treated with platinum-based chemotherapy.

Similar content being viewed by others
References
Jackman DM, Johnson BE (2005) Small-cell lung cancer. Lancet 366:1385–1396. https://doi.org/10.1016/S0140-6736(05)67569-1
Rocha Lima CM, Chiappori A (2003) Treatment of relapsed small-cell lung Cancer--a focus on the evolving role of Topotecan. Lung Cancer 40:229–236. https://doi.org/10.1016/s0169-5002(03)00039-4
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264. https://doi.org/10.1038/nrc3239
Horn L, Mansfield AS, Szczęsna A et al (2018) First-line Atezolizumab plus chemotherapy in extensive-stage small-cell lung Cancer. N Engl J Med 379:2220–2229. https://doi.org/10.1056/NEJMoa1809064
Paz-Ares L, Dvorkin M, Chen Y et al (2019) Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394:1929–1939. https://doi.org/10.1016/S0140-6736(19)32222-6
von Pawel J, Jotte R, Spigel DR et al (2014) Randomized phase III trial of Amrubicin versus Topotecan as second-line treatment for patients with small-cell lung Cancer. J Clin Oncol 32:4012–4019. https://doi.org/10.1200/JCO.2013.54.5392
Goto K, Ohe Y, Shibata T et al (2016) Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 17:1147–1157. https://doi.org/10.1016/S1470-2045(16)30104-8
Brody R, Zhang Y, Ballas M et al (2017) PD-L1 expression in advanced NSCLC: insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer 112:200–215. https://doi.org/10.1016/j.lungcan.2017.08.005
Chiang AC, Sequist LV, Gilbert J et al (2020) Clinical activity and safety of Atezolizumab in a phase 1 study of patients with relapsed/refractory small-cell lung Cancer. Clin Lung Cancer. https://doi.org/10.1016/j.cllc.2020.05.008
Funding
This study was supported by the Japan Society for the Promotion of Science (KAKENHI grant number 19 K16814).
Author information
Authors and Affiliations
Contributions
HI, KA: Concept and design, HI, KA, NM, TT: Care of patients, acquisition of data, HI, AK: Analysis and interpretation of data, HI, KA: Writing the original draft, HI, KA, AK, NM, TT, TH: Review and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Ishii reports grants and personal fees from Boerhinger-Ingelheim, personal fees from Ono Pharmaceutical, personal fees from Chugai Pharmaceutical, personal fees from Astra Zeneca, outside the submitted work. Dr. Azuma reports personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Ono Pharmaceutical, grants and personal fees from MSD Oncology, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Chugai Pharma, outside the submitted work. Dr. Kawahara has nothing to disclose. Dr. Matsuo reports personal fees from Boerhinger-Ingelheim, personal fees from Chugai Pharmaceutical, outside the submitted work. Dr. Tokito reports personal fees from Ono Pharmaceutical, personal fees from MSD Oncology, personal fees from Chugai Pharmaceutical, personal fees from Astra Zeneca, outside the submitted work. Dr. Hoshino reports grants from GSK Japan, grants from Novartis Pharmaceuticals Japan, grants from Chugai Pharmaceutical Co. Ltd., outside the submitted work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the declaration of Helsinki and ethical standards of the institutional research committee.
Consent to participate
Patient samples (anonymized) were taken as part of routine clinical care.
Consent for publication
Patient samples (anonymized) were taken as part of routine clinical care.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishii, H., Azuma, K., Kawahara, A. et al. Atezolizumab plus carboplatin and etoposide in small cell lung cancer patients previously treated with platinum-based chemotherapy. Invest New Drugs 39, 269–271 (2021). https://doi.org/10.1007/s10637-020-00983-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00983-6