Summary
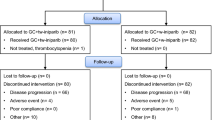
Background In the first part of this extensive phase I study (NCT00516724), continuous olaparib twice daily (bid) with carboplatin and/or paclitaxel resulted in myelosuppression and dose modifications. Here, we report the safety, tolerability, and efficacy of intermittent olaparib dosing combined with carboplatin and paclitaxel. Methods Patients with advanced solid tumors (part D) and enriched for ovarian and breast cancer (part E) received olaparib (capsule and tablet formulations) using intermittent schedules (2 to 10 days of a 21-day cycle) combined with carboplatin/paclitaxel. Safety assessments included evaluation of dose-limiting toxicities (DLTs; cycle 1 only), adverse events (AEs), and physical examinations. Pharmacokinetic assessments of olaparib capsule and tablet combined with carboplatin/paclitaxel were performed. Tumor responses (RECIST) were assessed every 2 cycles. Results In total, 132 heavily pre-treated patients were included. One DLT of grade 3 elevated alanine aminotransferase lasting for 8 days was reported (olaparib tablet 100 mg bid days 3–12, carboplatin area under the curve 4 and paclitaxel 175 mg/m2). The most common hematological AEs were neutropenia (47%) and thrombocytopenia (39%), which frequently led to dose modifications. Non-hematological AEs were predominantly grade 1–2, including alopecia (89%) and fatigue (84%). Overall objective response rate was 46%. Conclusions Discontinuous dosing of olaparib resulted in significant myelosuppression leading to dose interruptions and/or delays. Anti-tumor activity was encouraging in patients enriched with BRCA-mutated breast and ovarian cancer. The most appropriate olaparib tablet dose for use in further studies evaluating olaparib in combination with carboplatin and paclitaxel is 50 mg bid (days 1–5).

Similar content being viewed by others
References
Nguewa PA, Fuertes MA, Cepeda V, Alonso C, Quevedo C, Soto M, Pérez JM (2006) Poly(ADP-ribose) polymerase-1 inhibitor 3-aminobenzamide enhances apoptosis induction by platinum complexes in cisplatin-resistant tumor cells. Med Chem 2:47–53
Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, Derksen PW, de Bruin M, Zevenhoven J, Lau A, Boulter R, Cranston A, O'Connor MJ, Martin NM, Borst P, Jonkers J (2008) High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A 105:17079–17084
Friedlander M, Matulonis U, Gourley C, du Bois A, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T, Matei D, Shirinkin V, Selle F, Fielding A, Lowe ES, McMurtry EL, Spencer S, Rowe P, Mann H, Parry D, Ledermann J (2018) Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy. Br J Cancer 119:1075–1085
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira Frommer R, Safra T, Matei D, MacPherson E, Watkins C, Carmichael J, Matulonis U (2012) Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 366:1382–1392
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott CL, Meier W, Shapira-Frommer R, Safra T, Matei D, Fielding A, Spencer S, Dougherty B, Orr M, Hodgson D, Barrett JC, Matulonis U (2014) Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 15:852–861
Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, Korach J, Huzarski T, Poveda A, Pignata S, Friedlander M, Colombo N, Harter P, Fujiwara K, Ray-Coquard I, Banerjee S, Liu J, Lowe ES, Bloomfield R, Pautier P (2017) Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 18:1274–1284
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P (2017) Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377:523–533
Moore K, Colombo N, Scambia G, Kim B-G, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke GS, Gourley C, Banerjee S, Oza A, Gonzalez-Martin A, Aghajanian C, Bradley W, Mathews C, Liu J, Lowe ES, Bloomfield R, DiSilvestro P (2018) Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495–2505
FDA (2018) Lynparza prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208558s006lbl.pdf
European Medicines Agency (2018) Lynparza (olaparib) summary of product characteristics. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003726/human_med_001831.jsp&mid=WC0b01ac058001d124
van der Noll R, Ang JE, Jager A, Marchetti S, Mergui-Roelvink M, de Bono JS, Lolkema M, Brunetto A, , de Jonge MJ, van der Biessen D, Tchakov I, Bowen K, Schellens JHM, Arkenau H-T (2013) Phase I study of olaparib in combination with carboplatin and/or paclitaxel in patients with advanced solid tumors. J Clin Oncol 31(15 suppl):abst 2579
Gupta A, Moreno V, Dean EJ, Drew Y, Nicum S, Ranson M, Plummer R, Swaisland H, Burke W, McCormack P, Tchakov I, Middleton MR, Kaye SB, Molife LR (2012) Phase I study to determine the bioavailability and tolerability of a tablet formulation of the PARP inhibitor olaparib in patients with advanced solid tumors: dose-escalation phase. J Clin Oncol 30(15S):abst 3051
Mateo J, Moreno V, Gupta A, Kaye SB, Dean E, Middleton MR, Friedlander M, Gourley C, Plummer R, Rustin G, Sessa C, Leunen K, Ledermann J, Swaisland H, Fielding A, Bannister W, Nicum S, Molife LR (2016) An adaptive study to determine the optimal dose of the tablet formulation of the PARP inhibitor olaparib. Target Oncol 11:401–415
AstraZeneca (2016) Global policy: bioethics. Available at: https://www.astrazeneca.com/content/dam/az/PDF/2016/Bioethics_policy.pdf
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181
Rolfo C, Swaisland H, Leunen K, Rutten A, Soetekouw P, Slater S, Verheul HM, Fielding A, So K, Bannister W, Dean E (2015) Effect of food on the pharmacokinetics of olaparib after oral dosing of the capsule formulation in patients with advanced solid tumors. Adv Ther 32:510–522
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
ten Bokkel Huinink WW, van Warmerdam LJ, Helmerhorst TJ, Schaefers MC, Beijnen JH, Rodenhuis S (1997) Phase II study of the combination carboplatin and paclitaxel in patients with ovarian cancer. Ann Oncol 8:351–354
Balmana J, Tung NM, Isakoff SJ, Grana B, Ryan PD, Saura C, Lowe ES, Frewer P, Winer E, Baselga J, Garber JE (2014) Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann Oncol 25:1656–1663
Rajan A, Gutierrez M, Kummar S, Yancey M, Ji J, Simmons D, Parchment R, Tomaszewski J, Doroshow J, Giaccone G (2009) A phase I combination study of AZD2281 and cisplatin plus gemcitabine in adults with solid tumors. Ann Oncol 20(Suppl 3):iii42–iii43
Bendell J, O'Reilly EM, Middleton MR, Chau I, Hochster H, Fielding A, Burke W, Burris H III (2015) Phase I study of olaparib plus gemcitabine in patients with advanced solid tumours and comparison with gemcitabine alone in patients with locally advanced/metastatic pancreatic cancer. Ann Oncol 26:804–811
Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS, Colombo N, Spacek J, Vuylsteke P, Hirte H, Mahner S, Plante M, Schmalfeldt B, Mackay H, Rowbottom J, Lowe ES, Dougherty B, Barrett JC, Friedlander M (2015) Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 16:87–97
Verhagen CV, de Haan R, Hageman F, Oostendorp TP, Carli AL, O'Connor MJ, Jonkers J, Verheij M, van den Brekel MW, Vens C (2015) Extent of radiosensitization by the PARP inhibitor olaparib depends on its dose, the radiation dose and the integrity of the homologous recombination pathway of tumor cells. Radiother Oncol 116:358–365
Schouten PC, Dackus GM, Marchetti S, van Tinteren H, Sonke GS, Schellens JH, Linn SC (2016) A phase I followed by a randomized phase II trial of two cycles carboplatin-olaparib followed by olaparib monotherapy versus capecitabine in B. Trials 17:293
Acknowledgements
Nigel Baker of AstraZeneca is acknowledged for his support with the statistical analysis of this study. Editorial assistance was provided by Claire Routley, PhD, of Mudskipper Business Ltd., funded by AstraZeneca and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD).
Funding
This study was funded by AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ilian Tchakov was an employee of AstraZeneca at the time of this study. The other authors have no conflicts of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was performed in accordance with the Good Clinical Practice and the AstraZeneca policy on Bioethics.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and all applicable laws and regulations.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
van der Noll, R., Jager, A., Ang, J.E. et al. Phase I study of intermittent olaparib capsule or tablet dosing in combination with carboplatin and paclitaxel (part 2). Invest New Drugs 38, 1096–1107 (2020). https://doi.org/10.1007/s10637-019-00857-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00857-6