Summary
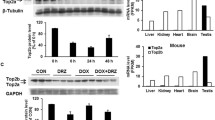
Purpose A novel doxorubicin (DOX) analog, 13-deoxy, 5-iminodoxorubicin (DIDOX), was synthesized to prevent quinone redox cycling and alcohol metabolite formation, two prevailing hypotheses of anthracycline cardiotoxicity. The chronic cardiotoxicity of DOX and DIDOX was compared. Since a recent hypothesis posits that DOX-induced chronic cardiotoxicity may be mediated by inhibition of the topoisomerase IIβ/DNA reaction, we also compared potency of DOX and DIDOX to inhibit topoisomerase IIβ decatenation of kinetoplast DNA (kDNA) (a series or interlocking small rings of DNA). Methods We compared DIDOX with DOX to alter cardiac function in a chronic rabbit model. We also compared potency to inhibit decatenation of kDNA by purified topoisomerase IIβ in vitro. Results DOX and DIDOX caused similar decreases in white and red blood cell counts indicating similar positions on the dose-response curve for cytotoxic efficacy. However, DOX but not DIDOX elicited a decrease in left ventricular fractional shortening and contractility of isolated left atrial preparations obtained at sacrifice. Histological scoring of apex and left ventricular free wall samples showed that DOX-treated rabbits had significantly more cardiac injury than samples from DIDOX or saline-treated rabbits. DOX inhibited decatenation of DNA by topoisomerase IIβ with an EC50 of 40.1 μM while DIDOX did not have any apparent effect on topoisomerase IIβ at the concentrations used in the study (0.1–100 μM). Conclusions Unlike DOX, DIDOX did not cause chronic cardiotoxicity and did not appear to interact with topoisomerase IIβ in decatenation assays consistent with the hypothesis that inhibition of the topoisomerase IIβ/DNA reaction may be a contributor of the mechanism of chronic DOX cardiotoxicity.





Similar content being viewed by others
References
Sawyer DB (2013) Anthracyclines and heart failure. N Engl J Med 368(12):1154–1156. doi:10.1056/NEJMcibr1214975
Vincent DT, Ibrahim YF, Espey MG, Suzuki YJ (2013) The role of antioxidants in the era of cardiooncology. Cancer Chemother Pharmacol 72(6):1157–1168. doi:10.1007/s00280-013-2260-4
Kalyanaraman B, Morehouse KM, Mason RP (1991) An electron paramagnetic resonance study of the interactions between the adriamycin semiquinone, hydrogen peroxide, iron-chelators, and radical scavengers. Arch Biochem Biophys 286(1):164–170
Vasquez-Vivar J, Martasek P, Hogg N, Masters BS, Pritchard KA Jr, Kalyanaraman B (1997) Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry 36(38):11293–11297. doi:10.1021/bi971475e
Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ, Ardehali H (2014) Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 124(2):617–630. doi:10.1172/JCI72931
Olson RD, Mushlin PS, Brenner DE, Fleischer S, Cusack BJ, Chang BK, Boucek RJ Jr (1988) Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proc Natl Acad Sci U S A 85(10):3585–3589
Minotti G, Recalcati S, Mordente A, Liberi G, Calafiore AM, Mancuso C, Preziosi P, Cairo G (1998) The secondary alcohol metabolite of doxorubicin irreversibly inactivates aconitase/iron regulatory protein-1 in cytosolic fractions from human myocardium. FASEB J 12(7):541–552
Minotti G, Recalcati S, Menna P, Salvatorelli E, Corna G, Cairo G (2004) Doxorubicin cardiotoxicity and the control of iron metabolism: quinone-dependent and independent mechanisms. Methods Enzymol 378:340–361. doi:10.1016/s0076-6879(04)78025-8
Gambliel HA, Burke BE, Cusack BJ, Walsh GM, Zhang YL, Mushlin PS, Olson RD (2002) Doxorubicin and C-13 deoxydoxorubicin effects on ryanodine receptor gene expression. Biochem Biophys Res Commun 291(3):433–438. doi:10.1006/bbrc.2002.6380
Kotamraju S, Chitambar CR, Kalivendi SV, Joseph J, Kalyanaraman B (2002) Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells: role of oxidant-induced iron signaling in apoptosis. J Biol Chem 277(19):17179–17187. doi:10.1074/jbc.M111604200
Kalivendi SV, Kotamraju S, Zhao H, Joseph J, Kalyanaraman B (2001) Doxorubicin-induced apoptosis is associated with increased transcription of endothelial nitric-oxide synthase. Effect of antiapoptotic antioxidants and calcium. J Biol Chem 276(50):47266–47276. doi:10.1074/jbc.M106829200
Hanson ES, Leibold EA (1999) Regulation of the iron regulatory proteins by reactive nitrogen and oxygen species. Gene Expr 7(4–6):367–376
Eisenstein RS (2000) Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annu Rev Nutr 20:627–662. doi:10.1146/annurev.nutr.20.1.627
Wardrop SL, Watts RN, Richardson DR (2000) Nitrogen monoxide activates iron regulatory protein 1 RNA-binding activity by two possible mechanisms: effect on the [4Fe-4S] cluster and iron mobilization from cells. Biochemistry 39(10):2748–2758
Minotti G, Cavaliere AF, Mordente A, Rossi M, Schiavello R, Zamparelli R, Possati G (1995) Secondary alcohol metabolites mediate iron delocalization in cytosolic fractions of myocardial biopsies exposed to anticancer anthracyclines. Novel linkage between anthracycline metabolism and iron-induced cardiotoxicity. J Clin Invest 95(4):1595–1605. doi:10.1172/JCI117833
Minotti G, Ronchi R, Salvatorelli E, Menna P, Cairo G (2001) Doxorubicin irreversibly inactivates iron regulatory proteins 1 and 2 in cardiomyocytes: evidence for distinct metabolic pathways and implications for iron-mediated cardiotoxicity of antitumor therapy. Cancer Res 61(23):8422–8428
Minotti G, Cairo G, Monti E (1999) Role of iron in anthracycline cardiotoxicity: new tunes for an old song? FASEB J 13(2):199–212
Cusack BJ, Mushlin PS, Voulelis LD, Li X, Boucek RJ Jr, Olson RD (1993) Daunorubicin-induced cardiac injury in the rabbit: a role for daunorubicinol? Toxicol Appl Pharmacol 118(2):177–185
Shadle SE, Bammel BP, Cusack BJ, Knighton RA, Olson SJ, Mushlin PS, Olson RD (2000) Daunorubicin cardiotoxicity: evidence for the importance of the quinone moiety in a free-radical-independent mechanism. Biochem Pharmacol 60(10):1435–1444
Zhang S, Liu X, Bawa-Khalfe T, LS L, Lyu YL, Liu LF, Yeh ET (2012) Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 18(11):1639–1642. doi:10.1038/nm.2919
Vavrova A, Jansova H, Mackova E, Machacek M, Haskova P, Tichotova L, Sterba M, Simunek T (2013) Catalytic inhibitors of topoisomerase II differently modulate the toxicity of anthracyclines in cardiac and cancer cells. PLoS One 8 (10):e76676. doi:10.1371/journal.pone.0076676
Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, Liu LF (2007) Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res 67(18):8839–8846. doi:10.1158/0008-5472.CAN-07-1649
Zhu H, Sarkar S, Scott L, Danelisen I, Trush MA, Jia Z, Li YR (2016) Doxorubicin Redox Biology: Redox Cycling, Topoisomerase Inhibition, and Oxidative Stress. 2016 1 (3):10
Herman EH, Ferrans VJ (1986) Pretreatment with ICRF-187 provides long-lasting protection against chronic daunorubicin cardiotoxicity in rabbits. Cancer Chemother Pharmacol 16(2):102–106
Hasinoff BB, Herman EH (2007) Dexrazoxane: how it works in cardiac and tumor cells. Is it a prodrug or is it a drug? Cardiovasc Toxicol 7(2):140–144. doi:10.1007/s12012-007-0023-3
Holstein SA, Bigelow JC, Olson RD, Vestal RE, Walsh GM, Hohl RJ (2015) Phase I and pharmacokinetic study of the novel anthracycline derivative 5-imino-13-deoxydoxorubicin (GPX-150) in patients with advanced solid tumors. Investig New Drugs 33(3):594–602. doi:10.1007/s10637-015-0220-z
Hasinoff BB, Wu X, Patel D, Kanagasabai R, Karmahapatra S, Yalowich JC (2016) Mechanisms of action and reduced cardiotoxicity of Pixantrone; a topoisomerase II targeting agent with cellular selectivity for the topoisomerase IIalpha isoform. J Pharmacol Exp Ther 356(2):397–409. doi:10.1124/jpet.115.228650
Acknowledgments
The authors wish to acknowledge Mary J. Hicks, Mandy R. McKay and Billie McKown for their excellent technical support. This study was supported in part by Gem Pharmaceuticals LLC, Birmingham, AL 35244 and NIH grant R41HL/CA64986. This material is the result of work supported by resources from the Boise VA Medical Center, Boise, ID 83702.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author’s disclosure of potential conflicts of interest
Nicole E. Frank: None.
Barry J. Cusack: None.
Todd T. Talley: Research funding from Gem Pharmaceuticals, LLC.
Jerry M. Walsh: Consultant for Gem Pharmaceuticals, LLC.
Richard D. Olson: Consultant for Gem Pharmaceuticals, LLC.
Funding/support
The work was sponsored by Gem Pharmaceuticals, LLC Birmingham, AL with funding from NIH grant R41HL/CA64986.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Not applicable.
Rights and permissions
About this article
Cite this article
Frank, N.E., Cusack, B.J., Talley, T.T. et al. Comparative effects of doxorubicin and a doxorubicin analog, 13-deoxy, 5-iminodoxorubicin (GPX-150), on human topoisomerase IIβ activity and cardiac function in a chronic rabbit model. Invest New Drugs 34, 693–700 (2016). https://doi.org/10.1007/s10637-016-0388-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0388-x