Summary
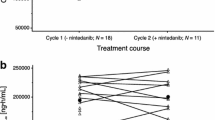
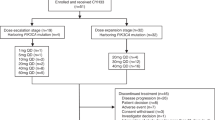
Pictilisib (GDC-0941) is an oral class I phosphatidylinositol-3-phosphate kinase inhibitor. This phase Ia/Ib study investigated the safety, tolerability, pharmacokinetics, and pharmacodynamics of pictilisib in monotherapy or in combination with carboplatin-paclitaxel and bevacizumab (CP + BEV) in Japanese patients with advanced solid tumors or non-squamous non-small cell lung cancer. A standard 3 + 3 dose escalation design was applied. In stage 1, 140, 260, or 340 mg/day of pictilisib was administered once daily to 12 patients with advanced solid tumors. In stage 2, 260 or 340 mg/day of pictilisib was administered in combination with CP + BEV to 7 patients with advanced non-squamous non-small cell lung cancer. In stage 1, 1 of 6 patients in the 340 mg/day cohort exhibited dose limiting toxicity (DLT) of grade 3 maculopapular rash. The maximum plasma concentration and area under the curve of pictilisib were dose-dependent. A reduction in phosphorylated AKT in platelet rich plasma was observed. No patient had an objective anti-tumor response. In stage 2, DLT was observed in 1 of 3 patients in the 260 mg/day cohort (grade 3 febrile neutropenia), and 2 of 4 patients in the 340 mg/day cohort (1 each of grade 3 febrile neutropenia and grade 3 febrile neutropenia/erythema multiforme). Partial responses were observed in 3 out of 7 patients. In conclusion, pictilisib was shown to have good safety and tolerability in Japanese patients with advanced solid tumors. A recommended dose of pictilisib in monotherapy was determined to be 340 mg once daily. For combination with CP + BEV, tolerability up to 260 mg/day was confirmed.






Similar content being viewed by others
References
Van Der Heijden MS, Bernards R (2010) Inhibition of the PI3K pathway: hope we can believe in? Clin Cancer Res 16:3094–3099
Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, Chuckowree IS, Clarke PA, Depledge P, Eccles SA, Friedman LS, Hayes A, Hancox TC, Kugendradas A, Lensun L, Moore P, Olivero AG, Pang J, Patel S, Pergl-Wilson GH, Raynaud FI, Robson A, Saghir N, Salphati L, Sohal S, Ultsch MH, Valenti M, Wallweber HJ, Wan NC, Wiesmann C, Workman P, Zhyvoloup A, Zvelebil MJ, Shuttleworth SJ (2008) The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem 51:5522–5532
Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, Schnell C, Guthy D, Nagel T, Wiesmann M, Brachmann S, Fritsch C, Dorsch M, Chène P, Shoemaker K, De Pover A, Menezes D, Martiny-Baron G, Fabbro D, Wilson CJ, Schlegel R, Hofmann F, García-Echeverría C, Sellers WR, Voliva CF (2012) Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther 11:317–328
Shapiro GI, Rodon J, Bedell C, Kwak EL, Baselga J, Braña I, Pandya SS, Scheffold C, Laird AD, Nguyen LT, Xu Y, Egile C, Edelman G (2014) Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245408 (XL147), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Clin Cancer Res 20:233–245
Raynaud FI, Eccles SA, Patel S, Alix S, Box G, Chuckowree I, Folkes A, Gowan S, De Haven Brandon A, Di Stefano F, Hayes A, Henley AT, Lensun L, Pergl-Wilson G, Robson A, Saghir N, Zhyvoloup A, McDonald E, Sheldrake P, Shuttleworth S, Valenti M, Wan NC, Clarke PA, Workman P (2009) Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther 8:1725–1738
Wallin JJ, Guan J, Prior WW, Lee LB, Berry L, Belmont LD, Koeppen H, Belvin M, Friedman LS, Sampath D (2012) GDC-0941, a novel class I selective PI3K inhibitor, enhances the efficacy of docetaxel in human breast cancer models by increasing cell death in vitro and in vivo. Clin Cancer Res 18:3901–3911
Sarker D, Ang JE, Baird R, Kristeleit R, Shah K, Moreno V, Clarke PA, Raynaud FI, Levy G, Ware JA, Mazina K, Lin R, Wu J, Fredrickson J, Spoerke JM, Lackner MR, Yan Y, Friedman LS, Kaye SB, Derynck MK, Workman P, de Bono JS (2015) First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res 21:77–86
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Niho S, Kunitoh H, Nokihara H, Horai T, Ichinose Y, Hida T, Yamamoto N, Kawahara M, Shinkai T, Nakagawa K, Matsui K, Negoro S, Yokoyama A, Kudoh S, Kiura K, Mori K, Okamoto H, Sakai H, Takeda K, Yokota S, Saijo N, Fukuoka M; JO19907 Study Group (2012) Randomized phase II study of first-line carboplatin-paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer 76:362–367
Zou ZQ, Zhang LN, Wang F, Bellenger J, Shen YZ, Zhang XH (2012) The novel dual PI3K/mTOR inhibitor GDC-0941 synergizes with the MEK inhibitor U0126 in non-small cell lung cancer cells. Mol Med Rep 5:503–508
Besse B, Soria J, Ware J, Adjei AA, Dy GK, Shankar G, Brachmann RK, Groen HJ (2011) A phase Ib study to evaluate the PI3-kinase inhibitor GDC-0941 with paclitaxel (P) and carboplatin (C), with and without bevacizumab (BEV), in patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2011 ASCO Annu Meet Abstr Part 1 29:3044
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1. Eur J Cancer 45:228–247
Besse B, Luft AV, Fadeeva N, Mezger J, Beck T, Bidoli P, Denis F, Paschold E, Robinet G, Groen H, Pawel JV, Lackner M, Gendreau S, Spoerke J, Bassett K, Koeppen H, Gilbert H, Jin H, Shankar G, Lin W, Felip E (2015) A phase II trial of pictilisib with chemotherapy in first-line non-squamous NSCLC. In: 16th World Conf. Lung Cancer. MINI30.12
Spigel DR, Luft A, Vynnychenko I, Fadeeva N, Mark Z, Ponce S, Matrosova M, Goldschmidt J, Szima B, Saleh M, Lackner M, Gendreau S, Bassett K, Spoerke J, Koeppen H, Gilbert H, Jin H, Shankar G, Lin W, Denis F (2015) A phase II trial of pictilisib with chemotherapy in first-line squamous NSCLC. In: 16th World Conf. Lung Cancer. MINI30.13
Dy GK, Adjei A, Groen HJM, Ware JA, Shankar G, Lin W, Besse B, Bahleda, R, Planchard D, Soria J (2013) A Phase Ib, open label, dose escalation study of the safety and pharmacology of PI3-Kinase (PI3K) Inhibitor GDC 0941 in combination with either Paclitaxel (Pac) and Carboplatin (Carbo) with or without Bevacizumab (Bev), or Pemetrexed (Pem), Cisplatin (Cis). 15th World Conf. Lung Cancer
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26:3543–3551
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan). Medical writing assistance and editing was provided by Macmillan Medical Communications (Tokyo, Japan), and funded by Chugai Pharmaceutical Co., Ltd.
Conflict of interest
Noboru Yamamoto has received research grants from Chugai Pharmaceutical, Eli Lilly, Taiho, Eisai, Quintiles, Astellas Pharma, Bristol-Myers Squibb, Daiichi Sankyo, Pfizer, Boehringer-Ingelheim, Kyowa-Hakko Kirin, and Bayer.
Yutaka Fujiwara has received research grants from AstraZeneca, Daiichi Sankyo, Chugai Pharmaceutical, Eisai, Eli Lilly, MSD, and Merck Serono.
Kenji Tamura has received research grants from Chugai Pharmaceutical, AstraZeneca, Daiichi Sankyo, Eisai, MSD, Eli Lilly, Pfizer, AbbVie and Byer.
Shunsuke Kondo has received research grants from Bayer, AstraZeneca, and Eli Lilly.
Satoru Iwasa has no conflicts of interest to declare.
Yuko Tanabe has no conflicts of interest to declare.
Atsushi Horiike has no conflicts of interest to declare.
Noriko Yanagitani is an advisory board member of Chugai Pharmaceutical.
Satoru Kitazono has no conflicts of interest to declare.
Michiyasu Inatani and Jun Tanaka are employee of Chugai Pharmaceutical.
Makoto Nishio has received research grants from Novartis, Ono Pharmaceutical, Chugai Pharmaceutical, Bristol-Myers Squibb, Taiho Pharmaceutical, Eli Lilly, Pfizer, Astellas Pharma, and AstraZeneca, and honoraria from Pfizer, Bristol-Myers Squibb, Ono Pharmaceutical, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, and AstraZeneca.
Human and animal rights
The study was conducted in accordance with the Declaration of Helsinki, the Pharmaceutical Affairs Law, and Good Clinical Practice. The study protocol was approved by the Institutional Review Boards of the participating institutions.
Informed consent
Written informed consent was obtained from all patients included in the study.
Additional information
This study is registered with the Japan Pharmaceutical Information Center, number JapicCTI-132162.
Rights and permissions
About this article
Cite this article
Yamamoto, N., Fujiwara, Y., Tamura, K. et al. Phase Ia/Ib study of the pan-class I PI3K inhibitor pictilisib (GDC-0941) administered as a single agent in Japanese patients with solid tumors and in combination in Japanese patients with non-squamous non-small cell lung cancer. Invest New Drugs 35, 37–46 (2017). https://doi.org/10.1007/s10637-016-0382-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0382-3