Summary
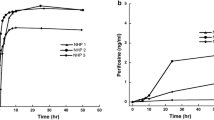
Purpose Vincristine sulfate liposomes injection (VSLI, Marqibo®) is an FDA approved encapsulated preparation of standard vincristine in sphingomyelin/cholesterol liposomes. Clinical pharmacokinetics show VSLI to be a long-circulating, slow release formulation that is confined to plasma, and prior data on cerebrospinal fluid (CSF) pharmacokinetics are lacking. We report our results comparing CSF and plasma pharmacokinetic parameters of intravenous aqueous vincristine to intravenous VSLI using an established non-human primate (NHP) model. Methods Three adult male rhesus monkeys (Macaca mulatta) were administered 0.1 mg/kg (1.2 mg/m2 human-equivalent dose) of vincristine or VSLI in a crossover pharmacokinetic study. Serial paired blood and CSF samples were obtained before infusion, at the end of infusion (EOI) and at various time points thereafter. Results In contrast to standard vincristine, which had a multi-exponential plasma disappearance curve with a median initial (EOI to 30 min post-infusion) half-life (T1/2) of 4.8 min (range, 4.4–5.0 min) and terminal T1/2 of 24.3 h, a near-monoexponential curve with a median T1/2 of 17.9 h (range, 13.9–21.5 h) hours was calculated with VSLI. The ratios Cl VCR:Cl VSLI for the individual NHP were 300, 463 and 477. Vincristine was not detected in any CSF sample after administration of either formulation. Conclusions In three animals, each serving as their own control, we demonstrate that the pharmacokinetic profile of VSLI shows markedly prolonged clearance (approximately 400-fold lower) of total vincristine in comparison to the standard aqueous formulation, enhancing our understanding of VSLI pharmacokinetics. Several clinical trials incorporating VSLI as substitution for standard vincristine are in progress.


Similar content being viewed by others
References
Lew G, Yanofsky R, Winick N, Rheingold S, Jorstad D, Lu X, Devidas M, Whitlock J, Hunger SP, Carroll WL (2011) Intensive vincristine is not feasibile in intermediate-risk relapse of childhood acute lymphoblastic leukemia (ALL): a report from children’s oncology group study AALL0433 (American society of peiatric hematology/oncology 25th annual meeting abstract, #159). Pediatr Blood Cancer 58(7):919
Webb MS, Harasym TO, Masin D, Bally MB, Mayer LD (1995) Sphingomyelin-cholesterol liposomes significantly enhance the pharmacokinetic and therapeutic properties of vincristine in murine and human tumour models. Br J Cancer 72(4):896–904
Webb MS, Logan P, Kanter PM, St-Onge G, Gelmon K, Harasym T, Mayer LD, Bally MB (1998) Preclinical pharmacology, toxicology and efficacy of sphingomyelin/cholesterol liposomal vincristine for therapeutic treatment of cancer. Cancer Chemother Pharmacol 42(6):461–470
Krishna R, Webb MS, St Onge G, Mayer LD (2001) Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J Pharmacol Exp Ther 298(3):1206–1212
U.S. Food and Drug Administration, FDA News Release, Aug. 9, 2012. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm315027.htm.
O’Brien S, Schiller G, Lister J, Damon L, Goldberg S, Aulitzky W, Ben-Yehuda D, Stock W, Coutre S, Douer D, Heffner LT, Larson M, Seiter K, Smith S, Assouline S, Kuriakose P, Maness L, Nagler A, Rowe J, Schaich M, Shpilberg O, Yee K, Schmieder G, Silverman JA, Thomas D, Deitcher SR, Kantarjian H (2013) High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult philadelphia chromosome-negative acute lymphoblastic leukemia. J Clin Oncol 31(6):676–683. doi:10.1200/JCO.2012.46.2309
Silverman JA, Deitcher SR (2013) Marqibo(R) (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol 71(3):555–564. doi:10.1007/s00280-012-2042-4
Silverman JA, Reynolds L, Deitcher SR (2013) Pharmacokinetics and pharmacodynamics of vincristine sulfate liposome injection (VSLI) in adults with acute lymphoblastic leukemia. J Clin Pharmacol 53(11):1139–1145. doi:10.1002/jcph.155
Kanter PM, Klaich GM, Bullard GA, King JM, Bally MB, Mayer LD (1994) Liposome encapsulated vincristine: preclinical toxicologic and pharmacologic comparison with free vincristine and empty liposomes in mice, rats and dogs. Anti-Cancer Drugs 5(5):579–590
McCully CL, Balis FM, Bacher J, Phillips J, Poplack DG (1990) A rhesus monkey model for continuous infusion of drugs into cerebrospinal fluid. Lab Anim Sci 40(5):520–525
Institute for Laboratory Animal Research (2011) Guide for the care and use of laboratory animals teW. National Academies Press, DC
Wood JH, Poplack DG, Bleyer WA, Ommaya AK (1977) Primate model for long-term study of intraventricularly or intrathecally administered drugs and intracranial pressure. Science 195(4277):499–501
Hagemeister F, Rodriguez MA, Deitcher SR, Younes A, Fayad L, Goy A, Dang NH, Forman A, McLaughlin P, Medeiros LJ, Pro B, Romaguera J, Samaniego F, Silverman JA, Sarris A, Cabanillas F (2013) Long term results of a phase 2 study of vincristine sulfate liposome injection (marqibo((R))) substituted for non-liposomal vincristine in cyclophosphamide, doxorubicin, vincristine, prednisone with or without rituximab for patients with untreated aggressive non-hodgkin lymphomas. Br J Haematol 162(5):631–638. doi:10.1111/bjh.12446
Sethi VS, Jackson Jr DV, White DR, Richards 2nd F, Stuart JJ, Muss HB, Cooper MR, Spurr CL (1981) Pharmacokinetics of vincristine sulfate in adult cancer patients. Cancer Res 41(9 Pt 1):3551–3555
Sethi VS, Kimball JC (1981) Pharmacokinetics of vincristine sulfate in children. Cancer Chemother Pharmacol 6(2):111–115
Kellie SJ, Barbaric D, Koopmans P, Earl J, Carr DJ, de Graaf SS (2002) Cerebrospinal fluid concentrations of vincristine after bolus intravenous dosing: a surrogate marker of brain penetration. Cancer 94(6):1815–1820
Yang F, Wang H, Liu M, Hu P, Jiang J (2013) Determination of free and total vincristine in human plasma after intravenous administration of vincristine sulfate liposome injection using ultra-high performance liquid chromatography tandem mass spectrometry. J Chromatogr A 1275:61–69. doi:10.1016/j.chroma.2012.12.026
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Nirali N. Shah and Diane E. Cole are co-first authors.
Rights and permissions
About this article
Cite this article
Shah, N.N., Cole, D.E., Lester-McCully, C.M. et al. Plasma and cerebrospinal fluid pharmacokinetics of vincristine and vincristine sulfate liposomes injection (VSLI, marqibo®) after intravenous administration in Non-human primates. Invest New Drugs 34, 61–65 (2016). https://doi.org/10.1007/s10637-015-0311-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0311-x