Summary
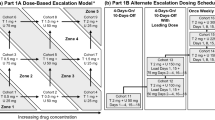
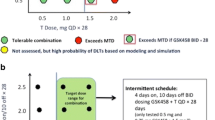
Background XL281 is a potent and selective inhibitor of wild-type and mutant RAF kinases with anti-tumor activity in multiple xenograft models. Mutations in KRAS or BRAF can activate the RAF/MEK/ERK pathway in human tumors and may confer sensitivity to RAF kinase inhibitors. Methods We performed a phase I study of XL281 in patients with advanced solid tumors. Patients were enrolled in successive cohorts of XL281 orally once daily in 28-day cycles. Twice daily dosing, different formulations, and the effect of food and famotidine were also studied. At the MTD expanded cohorts with defined mutations were treated. Results In total, 160 patients were treated. The MTD on the QD schedule was 150 mg. The most common toxicities were diarrhea, nausea, and fatigue. Plasma Cmax and AUC increased with dose. Famotidine resulted in lower AUC while food had no effect. Two patients had partial responses by RECIST: One with papillary thyroid cancer with NRAS mutation and one with uveal melanoma. Another nine patients had tumor decrease of >10 % but did not meet RECIST criteria for PR. Matched tumors pairs from 33 patients showed evidence of RAF inhibition with significant decreases in pERK, pMEK and pAKT. Conclusions XL281 was generally well tolerated and the MTD was established at 150 mg/day. Partial responses and clinical benefit were observed in several patients. Tumor biopsies demonstrated effective target inhibition.



Similar content being viewed by others
References
Kolch W, Kotwaliwale A, Vass K, Janosch P (2002) The role of Raf kinases in malignant transformation. Expert Rev Mol Med 4(8):1–18. doi:10.1017/S1462399402004386
Sebolt-Leopold JS, Herrera R (2004) Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer 4(12):937–947. doi:10.1038/nrc1503
Bos JL (1989) ras oncogenes in human cancer: a review. Cancer Res 49(17):4682–4689
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA (2002) Mutations of the BRAF gene in human cancer. Nature 417(6892):949–954. doi:10.1038/nature00766
Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL, Van Belle P, Elder DE, Herlyn M (2003) Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res 63(4):756–759
Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, Beller U, Westra WH, Ladenson PW, Sidransky D (2003) BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 95(8):625–627
Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA (2003) High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63(7):1454–1457
Schwartz GK, Robertson S, Shen A, Wang E, Pace L, Dials H, Mendelson D, Shannon P, Gordon M (2009) A phase I study of XL281, a selective oral RAF kinase inhibitor, in patients with advanced solid tumors. J Clin Oncol 27(15s):abstr 3513
Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, Hersey P, Kefford R, Lawrence D, Puzanov I, Lewis KD, Amaravadi RK, Chmielowski B, Lawrence HJ, Shyr Y, Ye F, Li J, Nolop KB, Lee RJ, Joe AK, Ribas A (2012) Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 366(8):707–714. doi:10.1056/NEJMoa1112302
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA, Group B-S (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364(26):2507–2516. doi:10.1056/NEJMoa1103782
Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr, Kaempgen E, Martin-Algarra S, Karaszewska B, Mauch C, Chiarion-Sileni V, Martin AM, Swann S, Haney P, Mirakhur B, Guckert ME, Goodman V, Chapman PB (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380(9839):358–365. doi:10.1016/S0140-6736(12)60868-X
Ascierto PA, Minor D, Ribas A, Lebbe C, O’Hagan A, Arya N, Guckert M, Schadendorf D, Kefford RF, Grob JJ, Hamid O, Amaravadi R, Simeone E, Wilhelm T, Kim KB, Long GV, Martin AM, Mazumdar J, Goodman VL, Trefzer U (2013) Phase II trial (BREAK-2) of the BRAF inhibitor Dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 31(26):3205–3211. doi:10.1200/JCO.2013.49.8691
Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, Burton EA, Wong B, Tsang G, West BL, Powell B, Shellooe R, Marimuthu A, Nguyen H, Zhang KY, Artis DR, Schlessinger J, Su F, Higgins B, Iyer R, D’Andrea K, Koehler A, Stumm M, Lin PS, Lee RJ, Grippo J, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, Chapman PB, Flaherty KT, Xu X, Nathanson KL, Nolop K (2010) Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467(7315):596–599. doi:10.1038/nature09454
Conflict of interest
Two authors (D.O.C. and S.H.J.) are paid employees of the sponsor. All other authors declare no potential conflicts of interest.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
The study was sponsored by Exelixis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dickson, M.A., Gordon, M.S., Edelman, G. et al. Phase I study of XL281 (BMS-908662), a potent oral RAF kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs 33, 349–356 (2015). https://doi.org/10.1007/s10637-014-0191-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0191-5