Summary
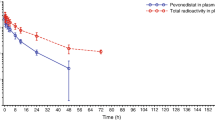
Background ES-285 (Spisulosine) is a novel marine compound with antitumor activity in preclinical studies. A phase I study was performed in patients with advanced solid tumors to determine the maximum tolerated dose (MTD), establish a safety profile, and to evaluate pharmacokinetics and efficacy of the drug. Patients and methods Thirty patients from two centers were treated with a three-hour ES-285 intravenous infusion for five consecutive days, every 3 weeks. Eleven dose levels were explored. Results No dose-limiting toxicity (DLT) occurred from 2 to 81 mg/m²/day. Three patients had DLT, one each at dose levels 160, 120 and 100 mg/m²/day; all had grade 4 transaminase increases, one of whom (160 mg/m²/day) had concomitant grade 4 hepatitis and grade 3 bilirubin elevation. The MTD of this regimen was not reached due to early termination of the ES-285 phase I program, but was considered to be 80 to 100 mg/m²/day. Other toxicities included mild to moderate asthenia, nausea, vomiting, anemia, lymphopenia, and injection site reaction. Pharmacokinetic analyses showed dose proportionality on Days 1 and 5, a wide distribution and a long half-life. Seven patients (five with colorectal cancer) had stable disease (1.2–4.1 months), lasting for more than 3 months in three patients. Conclusions Liver enzyme elevations were dose limiting for ES-285 in this administration schedule. Low antitumor activity was observed.

Similar content being viewed by others
References
Faircloth G, Cuevas C (2006) Kahalalide F and ES285: potent anticancer agents from marine molluscs. Prog Mol Subcell Biol 43:363–379
Baird R, Clarke P, Workman P (2005) ES-285, a novel marine anticancer agent: Investigation of mechanism of action using gene expression microarrays. AACR Meeting Abstracts 2005 (1):970
Sanchez AM, Malagarie-Cazenave S, Olea N, Vara D, Cuevas C, Diaz-Laviada I (2008) Spisulosine (ES-285) induces prostate tumor PC-3 and LNCaP cell death by de novo synthesis of ceramide and PKCzeta activation. Eur J Pharmacol 584(2–3):237–245. doi:S0014-2999(08)00158-1
Cuadros R, Montejo de Garcini E, Wandosell F, Faircloth G, Fernandez-Sousa JM, Avila J (2000) The marine compound spisulosine, an inhibitor of cell proliferation, promotes the disassembly of actin stress fibers. Cancer Lett 152(1):23–29. doi:S0304-3835(99)00428-0
Salcedo M, Cuevas C, Alonso JL, Otero G, Faircloth G, Fernandez-Sousa JM, Avila J, Wandosell F (2007) The marine sphingolipid-derived compound ES 285 triggers an atypical cell death pathway. Apoptosis 12(2):395–409. doi:10.1007/s10495-006-0573-z
Baird RD, Kitzen J, Clarke PA, Planting A, Reade S, Reid A, Welsh L, Lopez Lazaro L, de las Heras B, Judson IR, Kaye SB, Eskens F, Workman P, deBono JS, Verweij J (2009) Phase I safety, pharmacokinetic, and pharmacogenomic trial of ES-285, a novel marine cytotoxic agent, administered to adult patients with advanced solid tumors. Mol Cancer Ther 8(6):1430–1437. doi:1535-7163.MCT-08-1167
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Den Brok MW, Nuijen B, Meijer DM, Millan E, Manada C, Beijnen JH (2005) Pharmaceutical development of a parenteral lyophilised formulation of the investigational anticancer agent ES-285.HCL. PDA J Pharm Sci Technol 59(4):246–257
Den Brok MW, Nuijen B, Garcia JL, Miranda E, Calvo P, Manada C, Beijnen JH (2006) Compatibility and stability of the novel anticancer agent ES-285 x HCl formulated with 2-hydroxypropyl-beta-cyclodextrin in infusion devices. Pharmazie 61(1):21–24
Stokvis E, Nan-Offeringa L, Rosing H, Lopez-Lazaro L, Acena JL, Miranda E, Lyubimov A, Levine BS, D’Aleo C, Schellens JH, Beijnen JH (2003) Quantitative analysis of ES-285, an investigational marine anticancer drug, in human, mouse, rat, and dog plasma using coupled liquid chromatography and tandem mass spectrometry. J Mass Spectrom 38(5):548–554. doi:10.1002/jms.469
Salazar R, Soria J, Majem M, Ropert S, Garcia M, Armand J, Lopez Lazaro L, Escolar L, de las Heras B (2006) Phase I (3 hour infusion every 3 weeks) escalating dose study of intravenous ES-285 in patients with advanced malignant solid tumors. 4th International Symposium on Targeted Anticancer Therapies. Ann Oncol 17 (S3):iii49-iii50. doi:10.1093/annonc/md1922
Massard C, Majem M, Soria J-C, Salazar R, Deutsch E, Garcia M, Oaknin A, Soto A, de las Heras B, Armand J-P (2007) Phase I (3 hour infusion every 3 weeks) escalating dose study of intravenous es-285 in patients with advanced malignant solid tumors. AACR Meeting Abstracts (3_Molecular_Targets_Meeting):A4
Acknowledgements
We acknowledge the contribution of Sarah MacKenzie for medical writing support. We are grateful to the patients who participated in this trial, the many clinical research associates, nurses, and data managers for their help in patient recruitment, data management, and patient care during the conduct of this trial.
Disclosure
Employment or Leadership Position: H. Singer, PharmaMar; JL. Iglesias, PharmaMar; M. Cullell-Young, PharmaMar; Consultant or Advisory Role: P. Schöffski, PharmaMar; Stock Ownership: H. Singer, Zeltia Group; JL Iglesias, Zeltia Group; Honoraria: None; Research Funding: Patrick Schöffski, PharmaMar; Expert Testimony: Patrick Schöffski, PharmaMar; Other Remuneration: None.
Funding
This work was supported by PharmaMar SA, Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vilar, E., Grünwald, V., Schöffski, P. et al. A phase I dose-escalating study of ES-285, a marine sphingolipid-derived compound, with repeat dose administration in patients with advanced solid tumors. Invest New Drugs 30, 299–305 (2012). https://doi.org/10.1007/s10637-010-9529-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9529-9