Abstract
Purpose
Optic nerve crush (ONC) and subsequent axonal damage can be used in rodents to study the mechanism of retinal ganglion cell (RGC) degeneration. Here, we examined electroretinograms (ERGs) in post-ONC mice to investigate changes in the positive scotopic threshold response (pSTR). We then compared these changes with molecular and morphological changes to identify early objective biomarkers of RGC dysfunction.
Methods
Fifty 12-week-old C57BL/6 mice were included. ONC was used to induce axonal injury in the right eye of each animal, with the left eye used as a control. The expression of the RGC markers Brn3a and Brn3b was measured on days 1, 2, 3, 5 and 7 after ONC with quantitative real-time PCR. ERGs were recorded under dark adaptation with the stimulus intensity increasing from −6.2 to 0.43 log cd-s/m2 on days 1, 2, 3, 5, 7 and 10 after ONC. The pSTR, a- and b-wave amplitudes were measured. Inner retinal thickness around the optic nerve head was measured with spectral-domain optical coherence tomography on days 0, 2, 5, 7 and 10 after ONC.
Results
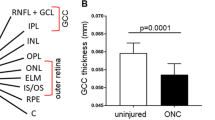
The expression of Brn3a and Brn3b began to significantly decrease on day 1 and day 2, respectively (P < 0.01). The amplitude of the pSTR underwent rapid, significant deterioration on day 3, after which it fell gradually (P < 0.01), while the a- and b-wave amplitudes remained unchanged throughout the experiment. Inner retinal thickness gradually decreased, with the most significant reduction on day 10 (P < 0.01).
Conclusions
Decrease in pSTR likely reflected the early loss of RGC function after ONC and that declining expression of RGC-specific genes preceded anatomical and functional changes in the RGCs.




Similar content being viewed by others
References
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial G (2002) Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 120(10):1268–1279
Collaborative Normal-Tension Glaucoma Study Group (1998) The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol 126(4):498–505
Musch DC, Gillespie BW, Lichter PR, Niziol LM, Janz NK, Investigators CS (2009) Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology 116(2):200–207. doi:10.1016/j.ophtha.2008.08.051
Ryu M, Yasuda M, Shi D, Shanab AY, Watanabe R, Himori N, Omodaka K, Yokoyama Y, Takano J, Saido T, Nakazawa T (2012) Critical role of calpain in axonal damage-induced retinal ganglion cell death. J Neurosci Res 90(4):802–815. doi:10.1002/jnr.22800
Himori N, Yamamoto K, Maruyama K, Ryu M, Taguchi K, Yamamoto M, Nakazawa T (2013) Critical role of Nrf2 in oxidative stress-induced retinal ganglion cell death. J Neurochem 127(5):669–680. doi:10.1111/jnc.12325
Fujita Y, Sato A, Yamashita T (2013) Brimonidine promotes axon growth after optic nerve injury through Erk phosphorylation. Cell Death Dis 4:e763. doi:10.1038/cddis.2013.298
Yasuda M, Tanaka Y, Ryu M, Tsuda S, Nakazawa T (2014) RNA sequence reveals mouse retinal transcriptome changes early after axonal injury. PLoS One 9(3):e93258. doi:10.1371/journal.pone.0093258
Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL 3rd (1999) The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40(6):1124–1136
Viswanathan S, Frishman LJ, Robson JG, Walters JW (2001) The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42(2):514–522
Gotoh Y, Machida S, Tazawa Y (2004) Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol 122(3):341–346. doi:10.1001/archopht.122.3.341
Rangaswamy NV, Frishman LJ, Dorotheo EU, Schiffman JS, Bahrani HM, Tang RA (2004) Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci 45(10):3827–3837. doi:10.1167/iovs.04-0458
Miyata K, Nakamura M, Kondo M, Lin J, Ueno S, Miyake Y, Terasaki H (2007) Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci 48(2):820–824. doi:10.1167/iovs.06-0845
Machida S, Gotoh Y, Toba Y, Ohtaki A, Kaneko M, Kurosaka D (2008) Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci 49(5):2201–2207. doi:10.1167/iovs.07-0887
Wang J, Cheng H, Hu YS, Tang RA, Frishman LJ (2012) The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci 53(3):1315–1323. doi:10.1167/iovs.11-8461
Machida S, Raz-Prag D, Fariss RN, Sieving PA, Bush RA (2008) Photopic ERG negative response from amacrine cell signaling in RCS rat retinal degeneration. Invest Ophthalmol Vis Sci 49(1):442–452. doi:10.1167/iovs.07-0291
Bui BV, Fortune B (2004) Ganglion cell contributions to the rat full-field electroretinogram. J Physiol 555(Pt 1):153–173. doi:10.1113/jphysiol.2003.052738
Sieving PA, Frishman LJ, Steinberg RH (1986) Scotopic threshold response of proximal retina in cat. J Neurophysiol 56(4):1049–1061
Frishman LJ, Shen FF, Du L, Robson JG, Harwerth RS, Smith EL 3rd, Carter-Dawson L, Crawford ML (1996) The scotopic electroretinogram of macaque after retinal ganglion cell loss from experimental glaucoma. Invest Ophthalmol Vis Sci 37(1):125–141
Mojumder DK, Sherry DM, Frishman LJ (2008) Contribution of voltage-gated sodium channels to the b-wave of the mammalian flash electroretinogram. J physiol 586(10):2551–2580. doi:10.1113/jphysiol.2008.150755
Smith BJ, Wang X, Chauhan BC, Cote PD, Tremblay F (2014) Contribution of retinal ganglion cells to the mouse electroretinogram. Doc Ophthalmol Adv Ophthalmol. doi:10.1007/s10633-014-9433-2
Shanab AY, Nakazawa T, Ryu M, Tanaka Y, Himori N, Taguchi K, Yasuda M, Watanabe R, Takano J, Saido T, Minegishi N, Miyata T, Abe T, Yamamoto M (2012) Metabolic stress response implicated in diabetic retinopathy: the role of calpain, and the therapeutic impact of calpain inhibitor. Neurobiol Dis 48(3):556–567. doi:10.1016/j.nbd.2012.07.025
Alarcon-Martinez L, Aviles-Trigueros M, Galindo-Romero C, Valiente-Soriano J, Agudo-Barriuso M, Villa Pde L, Villegas-Perez MP, Vidal-Sanz M (2010) ERG changes in albino and pigmented mice after optic nerve transection. Vision Res 50(21):2176–2187. doi:10.1016/j.visres.2010.08.014
Kohzaki K, Vingrys AJ, Bui BV (2008) Early inner retinal dysfunction in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci 49(8):3595–3604. doi:10.1167/iovs.08-1679
Shi M, Kumar SR, Motajo O, Kretschmer F, Mu X, Badea TC (2013) Genetic interactions between Brn3 transcription factors in retinal ganglion cell type specification. PLoS One 8(10):e76347. doi:10.1371/journal.pone.0076347
Sajgo S, Ghinia MG, Shi M, Liu P, Dong L, Parmhans N, Popescu O, Badea TC (2014) Dre-cre sequential recombination provides new tools for retinal ganglion cell labeling and manipulation in mice. PLoS One 9(3):e91435. doi:10.1371/journal.pone.0091435
Liu Y, McDowell CM, Zhang Z, Tebow HE, Wordinger RJ, Clark AF (2014) Monitoring retinal morphologic and functional changes in mice following optic nerve crush. Invest Ophthalmol Vis Sci 55(6):3766–3774. doi:10.1167/iovs.14-13895
Porciatti V (2007) The mouse pattern electroretinogram. Doc Ophthalmol Adv Ophthalmol 115(3):145–153. doi:10.1007/s10633-007-9059-8
Acknowledgments
We thank Ms. Junko Sato for her technical assistance. This work was supported in part by JSPS KAKENHI Grants-in-Aid for Scientific Research B (T.N. 26293372) and for Challenging Exploratory Research (Y.T. 26670263 and T.N. 26670751). This study was also supported by the JST Center for Revitalization Promotion (Y.T. and T.N.).
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yukita, M., Machida, S., Nishiguchi, K.M. et al. Molecular, anatomical and functional changes in the retinal ganglion cells after optic nerve crush in mice. Doc Ophthalmol 130, 149–156 (2015). https://doi.org/10.1007/s10633-014-9478-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-014-9478-2