Abstract
Background
Tumor necrosis factor antagonists (TNFs) are effective for moderate–severe Crohn’s disease (CD). Approximately one-third of patients have primary-nonresponse to TNFs, which is reported to predict worse response to subsequent TNF therapy. However, this is based on treatment with subcutaneously (SC) administered, fixed-dose TNFs after failure of intravenously (IV) administered, weight-based TNFs. No study has specifically assessed the clinical and endoscopic effectiveness of IV TNFs following primary-nonresponse to SC TNFs. We hypothesize that IV, weight-based TNF dosing offers advantages over SC, fixed-dose TNFs and may be effective despite primary-nonresponse to previous SC fixed-dose TNFs.
Methods
This retrospective cohort study identified patients with moderate–severe CD with primary-nonresponse to one or more SC TNFs who subsequently received the IV TNF, infliximab for ≥ 12 weeks. We described baseline characteristics, and clinical, endoscopic and biochemical response to therapy.
Results
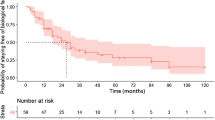
Key characteristics of 17 patients are described in Table 1. After ≥ 12 weeks of infliximab, 11 of 15 (73.3%) patients with clinical data reported clinical response and remission. Of 11 patients with endoscopic data, restaging colonoscopy revealed mucosal improvement in seven (63.6%) patients. Of these, five (45.5%) had endoscopic remission and three (27.3%) had mucosal healing.
Conclusions
Patients with moderate–severe CD with prior primary-nonresponse to SC, fixed-dose TNFs, subsequently treated with IV, weight-based TNF have high rates of clinical and endoscopic response and remission. Therefore, despite primary-nonresponse to SC TNFs, patients may benefit from IV TNF therapy and may not require a change to a different class of biologic therapy.


Similar content being viewed by others
References
Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239.
Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. quiz 591.
Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228–238.
Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–250.
Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838.
Gisbert JP, Marin AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41:613–623.
Christensen KR, Steenholdt C, Brynskov J. Clinical outcome of adalimumab therapy in patients with ulcerative colitis previously treated with infliximab: a Danish single-center cohort study. Scand J Gastroenterol. 2015;50:1018–1024.
Chaparro M, Andreu M, Barreiro-de Acosta M, et al. Effectiveness of infliximab after adalimumab failure in Crohn’s disease. World J Gastroenterol. 2012;18:5219–5224.
María del Carmen R, Chaparro M, Mesonero F, et al. Effectiveness of anti-TNFalpha drugs in patients with Crohn’s disease who do not achieve remission with their first anti-TNFalpha agent. Dig Liver Dis. 2016;48:613–619.
Scaldaferri F, D’Ambrosio D, Holleran G, et al. Body mass index influences infliximab post-infusion levels and correlates with prospective loss of response to the drug in a cohort of inflammatory bowel disease patients under maintenance therapy with Infliximab. PLoS ONE. 2017;12:e0186575.
Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther. 2016;43:317–333.
Kurti Z, Vegh Z, Golovics PA, Lakatos PL. Treat to target—lessons learnt. Dig Dis. 2016;34:147–152.
Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology. 2017;152:61–351.
Taleban S, Stewart KO, Li DK, et al. Clinical activity and quality of life indices are valid across ulcerative colitis but not Crohn’s disease phenotypes. Dig Dis Sci. 2016;61:2627–2635.
Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395.
Acknowledgments
Kindra Dawn Clark-Snustad, Anand Singla and Scott David Lee contributed equally to this article. All authors approved the final version of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study did not receive specific funding. Lee SD received grant and research support from the following: AbbVie Pharmaceuticals, UCB Pharma, Janssen Pharmaceuticals, Inc., Salix Pharmaceuticals, Takeda Pharmaceuticals, Inc., Celgene Pharmaceuticals, Inc., Pfizer Pharmaceuticals, Inc., Atlantic Pharmaceuticals, Ltd., Gilead Sciences, Inc., Tetherex Pharmaceuticals, Arena Pharmaceuticals, Shield Therapeutics PLC and is a consultant for UCB Pharma, Mesoblast, Cornerstones, Janssen Pharmaceuticals, Inc., Takeda Pharmaceuticals, Inc., Arena Pharmaceuticals, Eli Lilly and Company, Celgene Pharmaceuticals, Inc., Celltrion Healthcare Co, Ltd, Pfizer Pharmaceuticals, Salix Pharmaceuticals. Clark-Snustad KD has been a consultant for Takeda Pharmaceuticals, Inc. For the remaining authors, no conflicts of interest were declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Clark-Snustad, K.D., Singla, A. & Lee, S.D. Efficacy of Infliximab in Crohn’s Disease Patients with Prior Primary-Nonresponse to Tumor Necrosis Factor Antagonists. Dig Dis Sci 64, 1952–1958 (2019). https://doi.org/10.1007/s10620-019-05490-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05490-0