Abstract
Background
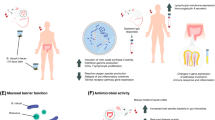
HIV progression is characterized by immune activation and microbial translocation from the gut. Short-chain fatty acids (SCFAs) are essential for gut homeostasis. Decreased intestinal SCFAs play a role in rapid HIV progression.
Aims
To compare the SCFA profile, intestinal microbiome, and intestinal mucosal injury between patients with HIV (but not AIDS) and healthy controls.
Methods
This was a prospective study of 15 patients without AIDS and 10 controls conducted between July 2016 and January 2017 at the Institute of Dermatology and Venereology (Sichuan Academy of Medical Sciences). Stool specimens were collected to analyze the microbiome and SCFAs. Blood I-FABP and d-lactate (gut injury markers) were measured as well as T cells in HIV-positive patients. Intestinal mucosa was observed by colonoscopy.
Results
Rikenellaceae, Microbacteriaceae, Roseburia, Lachnospiraceae, Alistipes, and Ruminococcaceae were decreased, while Moraxellaceae and Psychrobacter were increased in HIV-positive patients. Butyric acid (p = 0.04) and valeric acid (p = 0.03) were reduced in HIV-positive patients. Colonoscopy revealed no visible damage in all subjects. There were no differences in I-FABP and d-lactate between groups. Butyric and valeric acids mainly positively correlated with Rikenellaceae, Ruminococcaceae, Alistipes, Roseburia, and Lachnospiraceae. CD8+ cells were positively correlated with Proteobacteria. CD4+ cells, and CD4/CD8 were negatively correlated with acetic acid. CD8+ cells were positively correlated with valeric acid.
Conclusion
The differences in the distribution of intestinal flora between HIV-infected and healthy individuals, especially some SCFAs, suggest that there is already a predisposition to intestinal mucosa damage in HIV-infected individuals.








Similar content being viewed by others
References
Feinberg J. In the clinic. Management of newly diagnosed HIV infection. Ann Intern Med. 2011;155:ITC41.
Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 infection. N Engl J Med. 2011;364:1943–1954.
Wilcox CM, Saag MS. Gastrointestinal complications of HIV infection: changing priorities in the HAART era. Gut. 2008;57:861–870.
Li SX, Armstrong A, Neff CP, Shaffer M, Lozupone CA, Palmer BE. Complexities of gut microbiome dysbiosis in the context of HIV infection and antiretroviral therapy. Clin Pharmacol Ther. 2016;99:600–611.
Chevalier MF, Petitjean G, Dunyach-Remy C, et al. The Th17/Treg ratio, IL-1RA and sCD14 levels in primary HIV infection predict the T-cell activation set point in the absence of systemic microbial translocation. PLoS Pathog. 2013;9:e1003453.
Gori A, Tincati C, Rizzardini G, et al. Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J Clin Microbiol. 2008;46:757–758.
Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther. 2016;13:19.
Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Curr Opin Immunol. 2015;36:22–30.
Noguera-Julian M, Rocafort M, Guillen Y, et al. Gut microbiota linked to sexual preference and HIV infection. EBioMedicine. 2016;5:135–146.
Dillon SM, Kibbie J, Lee EJ, et al. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS. 2017;31:511–521.
Nowak P, Troseid M, Avershina E, et al. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS. 2015;29:2409–2418.
Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5:193ra191.
Zilberman-Schapira G, Zmora N, Itav S, Bashiardes S, Elinav H, Elinav E. The gut microbiome in human immunodeficiency virus infection. BMC Med. 2016;14:83.
Mutlu EA, Keshavarzian A, Losurdo J, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10:e1003829.
Saxena D, Li Y, Devota A, et al. Modulation of the orodigestive tract microbiome in HIV-infected patients. Oral Dis. 2016;22 Suppl 1:73–78.
Serrano-Villar S, Rojo D, Martinez-Martinez M, et al. Gut bacteria metabolism impacts immune recovery in HIV-infected individuals. EBioMedicine. 2016;8:203–216.
Tao JH, Duan JA, Jiang S, Guo JM, Qian YY, Qian DW. Simultaneous determination of six short-chain fatty acids in colonic contents of colitis mice after oral administration of polysaccharides from Chrysanthemum morifolium Ramat by gas chromatography with flame ionization detector. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1029–1030:88–94.
Das B, Dobrowolski C, Shahir AM, et al. Short chain fatty acids potently induce latent HIV-1 in T-cells by activating P-TEFb and multiple histone modifications. Virology. 2015;474:65–81.
Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671.
Kim CH, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277–288.
Yuille S, Reichardt N, Panda S, Dunbar H, Mulder IE. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE. 2018;13:e0201073.
Correa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol. 2016;5:e73.
Luhrs H, Gerke T, Muller JG, et al. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002;37:458–466.
Vazquez-Castellanos JF, Serrano-Villar S, Latorre A, et al. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. 2015;8:760–772.
Villar-Garcia J, Guerri-Fernandez R, Moya A, et al. Impact of probiotic Saccharomyces boulardii on the gut microbiome composition in HIV-treated patients: a double-blind, randomised, placebo-controlled trial. PLoS ONE. 2017;12:e0173802.
Ellis CL, Ma ZM, Mann SK, et al. Molecular characterization of stool microbiota in HIV-infected subjects by panbacterial and order-level 16S ribosomal DNA (rDNA) quantification and correlations with immune activation. J Acquir Immune Defic Syndr. 2011;57:363–370.
Ji Y, Zhang F, Zhang R, et al. Changes in intestinal microbiota in HIV-1-infected subjects following cART initiation: influence of CD4+ T cell count. Emerg Microbes Infect. 2018;7:113.
Wang X, Wang J, Rao B, Deng L. Gut flora profiling and fecal metabolite composition of colorectal cancer patients and healthy individuals. Exp Ther Med. 2017;13:2848–2854.
Zhou Y, Ou Z, Tang X, et al. Alterations in the gut microbiota of patients with acquired immune deficiency syndrome. J Cell Mol Med. 2018;22:2263–2271.
Travis AJ, Kelly D, Flint HJ, Aminov RI. Complete genome sequence of the human gut symbiont Roseburia hominis. Genome Announc. 2015;3:e01286–15.
Jost T, Lacroix C, Braegger C, Chassard C. Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br J Nutr. 2013;110:1253–1262.
Tamanai-Shacoori Z, Smida I, Bousarghin L, et al. Roseburia spp.: a marker of health? Future Microbiol. 2017;12:157–170.
Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275–1283.
Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30:1351–1360.
Hatziioanou D, Mayer MJ, Duncan SH, Flint HJ, Narbad A. A representative of the dominant human colonic Firmicutes, Roseburia faecis M72/1, forms a novel bacteriocin-like substance. Anaerobe. 2013;23:5–8.
Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–211.
Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8.
Meehan CJ, Beiko RG. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol Evol. 2014;6:703–713.
Reeves AE, Koenigsknecht MJ, Bergin IL, Young VB. Suppression of Clostridium difficile in the gastrointestinal tracts of germfree mice inoculated with a murine isolate from the family Lachnospiraceae. Infect Immun. 2012;80:3786–3794.
Sagheddu V, Patrone V, Miragoli F, Puglisi E, Morelli L. Infant early gut colonization by lachnospiraceae: high frequency of Ruminococcus gnavus. Front Pediatr. 2016;4:57.
Graf J. The Family Rikenellaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, eds. The Prokaryotes. Berlin: Springer; 2014.
Dziarski R, Park SY, Kashyap DR, Dowd SE, Gupta D. Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii enhance and Alistipes finegoldii attenuates colitis in mice. PLoS ONE. 2016;11:e0146162.
Zevin AS, McKinnon L, Burgener A, Klatt NR. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS. 2016;11:182–190.
Klase Z, Ortiz A, Deleage C, et al. Dysbiotic bacteria translocate in progressive SIV infection. Mucosal Immunol. 2015;8:1009–1020.
Teixeira LM, Merquior VLC. The Family Moraxellaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, eds. The Prokaryotes. Berlin: Springer; 2014.
Saito R, Nonaka S, Nishiyama H, Okamura N. Molecular mechanism of macrolide-lincosamide resistance in Moraxella catarrhalis. J Med Microbiol. 2012;61:1435–1438.
Alcala L, Ruiz-Serrano MJ, Cosin J, Garcia-Garrote F, Ortega A, Bouza E. Disseminated infection due to Mycobacterium simiae in an AIDS patient: case report and review. Clin Microbiol Infect. 1999;5:294–296.
Michelini Z, Baroncelli S, Fantauzzi A, et al. Reduced plasma levels of sCD14 and I-FABP in HIV-infected patients with mesalazine-treated ulcerative colitis. HIV Clin Trials. 2016;17:49–54.
Smith SM, Eng RH, Campos JM, Chmel H. d-lactic acid measurements in the diagnosis of bacterial infections. J Clin Microbiol. 1989;27:385–388.
Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270.
Monaco CL, Gootenberg DB, Zhao G, et al. Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquired immunodeficiency syndrome. Cell Host Microbe. 2016;19:311–322.
Tuddenham S, Sears CL. The intestinal microbiome and health. Curr Opin Infect Dis. 2015;28:464–470.
Farhour Z, Mehraj V, Chen J, Ramendra R, Lu H, Routy JP. Use of (1– > 3)-beta-d-glucan for diagnosis and management of invasive mycoses in HIV-infected patients. Mycoses. 2018;61:718–722.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 81101231) and the Basic Scientific Research Projects of Sichuan Provincial Scientific Research Institutes (no. 2017YSKY0001). The funders had no role in the study design, data collection and interpretation, or decision to submit the work for publication.
Funding
This study was supported by the National Natural Science Foundation of China (no. 81101231) and Basic Scientific Research Projects of Sichuan Provincial Scientific Research Institutes (no. 2017YSKY0001). The funders had no role in the study design, data collection and interpretation, or decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
YQ and HX carried out the experiments, participated in collecting data, and drafted the manuscript. CS and YW performed the statistical analysis and participated in its design. QY and QP helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
The study was approved by the ethics committee of the Sichuan Provincial People’s Hospital. All subjects provided written informed consent.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Qing, Y., Xie, H., Su, C. et al. Gut Microbiome, Short-Chain Fatty Acids, and Mucosa Injury in Young Adults with Human Immunodeficiency Virus Infection. Dig Dis Sci 64, 1830–1843 (2019). https://doi.org/10.1007/s10620-018-5428-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5428-2