Abstract
Background
There is uncertainty as to the appropriate follow-up of patients who test positive on multimarker stool DNA (sDNA) testing and have a colonoscopy without neoplasia.
Aims
To determine the prevalence of missed colonic or occult upper gastrointestinal neoplasia in patients with an apparent false positive sDNA.
Methods
We prospectively identified 30 patients who tested positive with a commercially available sDNA followed by colonoscopy without neoplastic lesions. Patients were invited to undergo repeat sDNA at 11–29 months after the initial test followed by repeat colonoscopy and upper endoscopy. We determined the presence of neoplastic lesions on repeat evaluation stratified by results of repeat sDNA.
Results
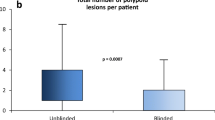
Twelve patients were restudied. Seven patients had a negative second sDNA test and a normal second colonoscopy and upper endoscopy. In contrast, 5 of 12 subjects had a persistently positive second sDNA test, and 3 had positive findings, including a 3-cm sessile transverse colon adenoma with high-grade dysplasia, a 2-cm right colon sessile serrated adenoma with dysplasia, and a nonadvanced colon adenoma (p = 0.045). These corresponded to a positive predictive value of 0.60 (95% CI 0.17–1.00) and a negative predictive value of 1.00 (95% CI 1.00–1.00) for the second sDNA test. In addition, the medical records of all 30 subjects with apparent false positive testing were reviewed and no documented cases of malignant tumors were recorded.
Conclusions
Repeat positive sDNA testing may identify a subset of patients with missed or occult colorectal neoplasia after negative colonoscopy for an initially positive sDNA. High-quality colonoscopy with careful attention to the right colon in patients with positive sDNA is critically important and may avoid false negative colonoscopy.

Similar content being viewed by others
References
US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA.. 2016;315:2564–2575.
Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multi-target stool DNA testing for colorectal cancer screening. N Engl J Med. 2014;370:1287–1297.
Heresbach D, Barrioz T, Lapalus MG, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284–290.
Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1375–1389.
Kisiel JB, Yab TC, Taylor WR, et al. Stool DNA testing for the detection of pancreatic cancer: assessment of methylation marker candidates. Cancer. 2012;118:2623–2631.
Cotter TG, Burger KN, Devens ME, et al. Long-term follow-up of patients having false positive multi-target stool DNA tests after negative screening colonoscopy. The LONG-HAUL cohort study. Cancer Epidemiol Biomark Prev. 2017;26:614–621.
FDA Summary of Safety and Effectiveness, PMA P130017b August 2014, page 9. www.fda.cov. Accessed 16.02.2018.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577.
Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol.. 2015;110:72–90.
Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105:2656–2664.
Kahi CJ, Hewett DG, Norton DL, et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–46.
Johnson DH, Kisiel JB, Burger KW, et al. Multitarget stool DNA test: clinical performance and impact on yield and quality of colonoscopy for colorectal cancer screening. Gastrointest Endosc. 2017;85:657–665.
Manfredi MA, Abu Dayyeh BK, Bhat YM, et al. Electronic chromoendoscopy. Gastrointest Endosc. 2015;81:249–261.
Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153:307–323.
Acknowledgments
This work was supported by the Case Gastrointestinal SPORE (P50CA150964), the Case Comprehensive Cancer Center (P30CA43703), the Cleveland Digestive Disease Research Core Center (P30DK097948), University of Michigan Gastrointestinal SPORE (P50CA130810), the University of Michigan Comprehensive Cancer Center (P30CA046592), the Early Detection Research Network (U01CA086400) and U01CA181770.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Barry M. Berger, MD, is a full-time employee of Exact Sciences, Inc, the manufacturer of the stool DNA technology used in this study. None of the other authors have conflicts of interest.
Rights and permissions
About this article
Cite this article
Cooper, G.S., Markowitz, S.D., Chen, Z. et al. Evaluation of Patients with an Apparent False Positive Stool DNA Test: The Role of Repeat Stool DNA Testing. Dig Dis Sci 63, 1449–1453 (2018). https://doi.org/10.1007/s10620-018-5001-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5001-z