Abstract
Introduction
Controlled attenuation parameter (CAP) is a new method for the diagnosis of steatosis. Until now, CAP was available only with the M probe of the Fibroscan. The aim of this study was to evaluate the diagnostic performance of CAP with the XL probe versus CAP with the M probe, using liver biopsy (LB) as gold standard.
Patients and Methods
A total of 236 patients with chronic liver disease undergoing LB had CAP measurement with M and XL probes the same day. All LB were analyzed independently by two experienced pathologists.
Results
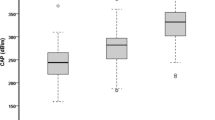
Median CAP was 240.5 and 239.5 dB/m with the M and XL probes, respectively. For the detection of steatosis grade with the M and XL probes, AUROCs were 0.82/0.83 for S ≥ 1, 0.89/0.88 for S ≥ 2, and 0.92/0.93 for S3, respectively. Cutoffs were (M and XL probes) 246/242 for S ≥ 1, 269/267 for S ≥ 2, and 285/286 dB/m for S3, respectively. The factor significantly associated with CAP with the M and XL probes was steatosis grade. In multivariate analysis, a low CAP value with XL probe was negatively associated with waist circumference, triglycerides, albumin, and the alcohol consumption, and positively with alkaline phosphatases. In multivariate analysis, a high CAP value with the XL probe was positively associated with waist circumference and triglycerides.
Conclusion
CAP with the XL probe is a new tool for the diagnosis of steatosis. This parameter could be useful for the diagnosis and the follow-up of obese patients.



Similar content being viewed by others
Abbreviations
- CAP:
-
Controlled attenuation parameter
- LB:
-
Liver biopsy
- AUROC:
-
Area under the receiver operating curve (ROC)
- NAFLD:
-
Nonalcoholic fatty liver disease
- BMI:
-
Body mass index
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- IQR:
-
Interquartile range
- LSM:
-
Liver stiffness measurement
References
Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131.
Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555.
Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835.
Chon YE, Jung KS, Kim SU, et al. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014;34:102–109.
de Ledinghen V, Vergniol J, Capdepont M, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026–1031.
de Ledinghen V, Wong GL, Vergniol J, et al. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:848–855.
Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–1030. doi:10.1016/j.jhep.2016.12.022.
Mi YQ, Shi QY, Xu L, et al. Controlled attenuation parameter for noninvasive assessment of hepatic steatosis using Fibroscan(R): validation in chronic hepatitis B. Dig Dis Sci. 2015;60:243–251.
Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13–20.
Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862–1871.
Sasso M, Audiere S, Kemgang A, et al. Liver steatosis assessed by controlled attenuation parameter (CAP) measured with the XL probe of the Fibroscan: a pilot study assessing diagnostic accuracy. Ultrasound Med Biol. 2016;42:92–103.
de Ledinghen V, Vergniol J. Transient elastography (Fibroscan). Gastroenterol Clin Biol. 2008;32:58–67.
Bedossa P, Poitou C, Veyrie N, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–1759.
Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41.
Team. RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2015. https://www.R-project.org/.
Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29:1470–1476.
Myers RP, Pollett A, Kirsch R, et al. Controlled attenuation parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902–910.
WHO. Obesity and overweight—fact sheet n°311. http://www.who.int/mediacentre/factsheets/fs311/en/: WHO; 2015.
Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:837–858.
Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, american college of gastroenterology, and the American gastroenterological association. Hepatology. 2012;55:2005–2023.
Xu C, Yu C, Ma H, Xu L, Miao M, Li Y. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai study. Am J Gastroenterol. 2013;108:1299–1304.
Author’s contributions
VL performed research, analyzed data, designed research study, and wrote paper. JV performed research and analyzed data. JH, WM, and PB performed research. VP performed research and designed research study. All authors approved the final version of the manuscript.
Funding
Funding for this study was obtained from Echosens (Paris).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Victor de Lédinghen: SuperSonic Imagine. Other authors: no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10620_2017_4638_MOESM1_ESM.tiff
Supplementary file Fig. 1. Controlled attenuation parameter (CAP) measured with the M probe versus the CAP measured with the XL probe
Rights and permissions
About this article
Cite this article
de Lédinghen, V., Hiriart, JB., Vergniol, J. et al. Controlled Attenuation Parameter (CAP) with the XL Probe of the Fibroscan®: A Comparative Study with the M Probe and Liver Biopsy. Dig Dis Sci 62, 2569–2577 (2017). https://doi.org/10.1007/s10620-017-4638-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4638-3