Abstract
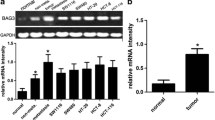
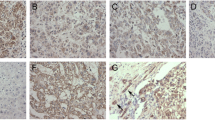
Tumor metastasis is the process by which tumor cells disseminate from tumors and enter nearby and distant microenvironments for new colonization. Bif-1 (BAX-interacting factor 1), which has a BAR domain and an SH3 domain, has been reported to be involved in cell growth, apoptosis and autophagy. However, the influence of Bif-1 on metastasis has been less studied. To understand the role of Bif-1 in metastasis, we studied the expression levels of Bif-1 in human HCC specimens using immunohistochemistry, a tissue microarray and quantitative PCR. The function of Bif-1 was assessed in migration and translocation assays and the pulmonary metastatic animal model. The relationship between Bif-1 and the Rho family was determined using immunoblot analyses and chromatin immunoprecipitation. The results showed that the expression of Bif-1 was higher in hepatocellular carcinoma (HCC) than matched adjacent non-tumor liver tissues. Increased Bif-1 expression was associated with tumor size and the intercellular spread and metastasis of HCC. Analysis of the relationship between Bif-1 expression and patients’ clinical characteristics revealed that patients with higher levels of Bif-1 had shorter disease-free and overall survival rates. Knockdown of Bif-1 with RNAi suppressed the migration of HCC cells and pulmonary metastasis and decreased the expression of Cdc42, a member of the Rho family. Bif-1 localized to the cytosol and nucleus and interacted with the promoter transcription region of Cdc42, which may regulate Cdc42 expression. Our results demonstrate a novel role of Bif-1 in HCC, in which Bif-1 promotes cell metastasis by regulating Cdc42 expression and activity.






Similar content being viewed by others
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- Bif-1:
-
BAX-interacting factor 1
- EMT:
-
Epithelial-mesenchymal transition
- BAR:
-
Bin/Amphiphysin/Rvs
- SH3:
-
Src homology 3
- Irgm1:
-
Immunity-related GTPase family member 1
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
References
Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM (2007) Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer 7(4):246–255
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890
Cuddeback SM, Yamaguchi H, Komatsu K, Miyashita T, Yamada M, Wu C, Singh S, Wang HG (2001) Molecular cloning and characterization of Bif-1. A novel Src homology 3 domain-containing protein that associates with Bax. J Biol Chem 276(23):20559–20565
Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mule JJ, Pledger WJ, Wang HG (2007) Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol 9(10):1142–1151
Takahashi Y, Meyerkord CL, Hori T, Runkle K, Fox TE, Kester M, Loughran TP, Wang HG (2011) Bif-1 regulates Atg9 trafficking by mediating the fission of Golgi membranes during autophagy. Autophagy 7(1):61–73
Runkle KB, Meyerkord CL, Desai NV, Takahashi Y, Wang HG (2012) Bif-1 suppresses breast cancer cell migration by promoting EGFR endocytic degradation. Cancer Biol Ther 13(10):956–966
Aramaki Y, Ogawa K, Toh Y, Ito T, Akimitsu N, Hamamoto H, Sekimizu K, Matsusue K, Kono A, Iguchi H, Takiguchi S (2005) Direct interaction between metastasis-associated protein 1 and endophilin 3. FEBS Lett 579(17):3731–3736
de Las Heras JI, Batrakou DG, Schirmer EC (2013) Cancer biology and the nuclear envelope: a convoluted relationship. Semin Cancer Biol 23(2):125–137
Kim SY, Oh YL, Kim KM, Jeong EG, Kim MS, Yoo NJ, Lee SH (2008) Decreased expression of Bax-interacting factor-1 (Bif-1) in invasive urinary bladder and gallbladder cancers. Pathology 40(6):553–557
Ko YH, Cho YS, Won HS, Jeon EK, An HJ, Hong SU, Park JH, Lee MA (2013) Prognostic significance of autophagy-related protein expression in resected pancreatic ductal adenocarcinoma. Pancreas 42(5):829–835
Ho J, Kong JW, Choong LY, Loh MC, Toy W, Chong PK, Wong CH, Wong CY, Shah N, Lim YP (2009) Novel breast cancer metastasis-associated proteins. J Proteome Res 8(2):583–594
Coppola D, Oliveri C, Sayegh Z, Boulware D, Takahashi Y, Pow-Sang J, Djeu JY, Wang HG (2008) Bax-interacting factor-1 expression in prostate cancer. Clin Genitourin Cancer 6(2):117–121
Lee JW, Jeong EG, Soung YH, Nam SW, Lee JY, Yoo NJ, Lee SH (2006) Decreased expression of tumour suppressor Bax-interacting factor-1 (Bif-1), a Bax activator, in gastric carcinomas. Pathology 38(4):312–315
Fan R, Miao Y, Shan X, Qian H, Song C, Wu G, Chen Y, Zha W (2012) Bif-1 is overexpressed in hepatocellular carcinoma and correlates with shortened patient survival. Oncol Lett 3(4):851–854
Schlauder SM, Calder KB, Khalil FK, Passmore L, Mathew RA, Morgan MB (2009) Bif-1 and Bax expression in cutaneous Merkel cell carcinoma. J Cutan Pathol 36(1):21–25
Wang T, Hu HS, Feng YX, Shi J, Li N, Guo WX, Xue J, Xie D, Liu SR, Wu MC, Cheng SQ (2010) Characterisation of a novel cell line (CSQT-2) with high metastatic activity derived from portal vein tumour thrombus of hepatocellular carcinoma. Br J Cancer 102(11):1618–1626
Han CP, Kok LF, Lee MY, Wu TS, Ruan A, Cheng YW, Wang PH, Koo CL, Tyan YS (2010) Five commonly used markers (p53, TTF1, CK7, CK20, and CK34betaE12) are of no use in distinguishing between primary endocervical and endometrial adenocarcinomas in a tissue microarray extension study. Arch Gynecol Obstet 281(2):317–323
Coppola D, Khalil F, Eschrich SA, Boulware D, Yeatman T, Wang HG (2008) Down-regulation of Bax-interacting factor-1 in colorectal adenocarcinoma. Cancer 113(10):2665–2670
Coppola D, Helm J, Ghayouri M, Malafa MP, Wang HG (2011) Down-regulation of Bax-interacting factor 1 in human pancreatic ductal adenocarcinoma. Pancreas 40(3):433–437
Etxebarria A, Terrones O, Yamaguchi H, Landajuela A, Landeta O, Antonsson B, Wang HG, Basanez G (2009) Endophilin B1/Bif-1 stimulates BAX activation independently from its capacity to produce large scale membrane morphological rearrangements. J Biol Chem 284(7):4200–4212
Dong H, Tian L, Li R, Pei C, Fu Y, Dong X, Xia F, Wang C, Li W, Guo X, Gu C, Li B, Liu A, Ren H, Xu H (2015) IFNg-induced Irgm1 promotes tumorigenesis of melanoma via dual regulation of apoptosis and Bif-1-dependent autophagy. Oncogene 34(42):5363–5371
Juhasz G, Hill JH, Yan Y, Sass M, Baehrecke EH, Backer JM, Neufeld TP (2008) The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol 181(4):655–666
Ku B, Woo JS, Liang C, Lee KH, Jung JU, Oh BH (2008) An insight into the mechanistic role of Beclin 1 and its inhibition by prosurvival Bcl-2 family proteins. Autophagy 4(4):519–520
Levine B, Sinha S, Kroemer G (2008) Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 4(5):600–606
Takahashi Y, Meyerkord CL, Wang HG (2008) BARgaining membranes for autophagosome formation: regulation of autophagy and tumorigenesis by Bif-1/Endophilin B1. Autophagy 4(1):121–124
Yamaguchi H, Woods NT, Dorsey JF, Takahashi Y, Gjertsen NR, Yeatman T, Wu J, Wang HG (2008) SRC directly phosphorylates Bif-1 and prevents its interaction with Bax and the initiation of anoikis. J Biol Chem 283(27):19112–19118
He S, Ni D, Ma B, Lee JH, Zhang T, Ghozalli I, Pirooz SD, Zhao Z, Bharatham N, Li B, Oh S, Lee WH, Takahashi Y, Wang HG, Minassian A, Feng P, Deretic V, Pepperkok R, Tagaya M, Yoon HS, Liang C (2013) PtdIns(3)P-bound UVRAG coordinates Golgi-ER retrograde and Atg9 transport by differential interactions with the ER tether and the beclin 1 complex. Nat Cell Biol 15(10):1206–1219
Khan MM, Strack S, Wild F, Hanashima A, Gasch A, Brohm K, Reischl M, Carnio S, Labeit D, Sandri M, Labeit S, Rudolf R (2014) Role of autophagy, SQSTM1, SH3GLB1, and TRIM63 in the turnover of nicotinic acetylcholine receptors. Autophagy 10(1):123–136
Xie T, Li SJ, Guo MR, Wu Y, Wang HY, Zhang K, Zhang X, Ouyang L, Liu J (2015) Untangling knots between autophagic targets and candidate drugs, in cancer therapy. Cell Prolif 48(2):119–139
Genda T, Sakamoto M, Ichida T, Asakura H, Kojiro M, Narumiya S, Hirohashi S (1999) Cell motility mediated by rho and Rho-associated protein kinase plays a critical role in intrahepatic metastasis of human hepatocellular carcinoma. Hepatology 30(4):1027–1036
Liu S, Goldstein RH, Scepansky EM, Rosenblatt M (2009) Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res 69(22):8742–8751
Kamai T, Tsujii T, Arai K, Takagi K, Asami H, Ito Y, Oshima H (2003) Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clin Cancer Res 9(7):2632–2641
Kusama T, Mukai M, Endo H, Ishikawa O, Tatsuta M, Nakamura H, Inoue M (2006) Inactivation of Rho GTPases by p190 RhoGAP reduces human pancreatic cancer cell invasion and metastasis. Cancer Sci 97(9):848–853
Li Z, Chang Z, Chiao LJ, Kang Y, Xia Q, Zhu C, Fleming JB, Evans DB, Chiao PJ (2009) TrkBT1 induces liver metastasis of pancreatic cancer cells by sequestering Rho GDP dissociation inhibitor and promoting RhoA activation. Cancer Res 69(19):7851–7859
Titus B, Schwartz MA, Theodorescu D (2005) Rho proteins in cell migration and metastasis. Crit Rev Eukaryot Gene Expr 15(2):103–114
Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang CK, Wu D (2003) Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell 114(2):215–227
Meili R, Sasaki AT, Firtel RA (2005) Rho Rocks PTEN. Nat Cell Biol 7(4):334–335
Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D (2005) Regulation of PTEN by Rho small GTPases. Nat Cell Biol 7(4):399–404
Iiizumi M, Bandyopadhyay S, Pai SK, Watabe M, Hirota S, Hosobe S, Tsukada T, Miura K, Saito K, Furuta E, Liu W, Xing F, Okuda H, Kobayashi A, Watabe K (2008) RhoC promotes metastasis via activation of the Pyk2 pathway in prostate cancer. Cancer Res 68(18):7613–7620
Acknowledgments
We thank Huanlin Sun, Dongping Hu, Liang Tang, LinnaGuo, and Shanna Huang for their technical assistance. This work was supported by Grants from the National Natural Science Foundation of China (81221061, 81272427 and 31471339); The Ministry of Science and Technology Key Program (2012ZX10002-009, -011); The State Key Project on Diseases of China (2012zx10002016016003); The China National Funds for Distinguished Young Scientists (81125018); the Chang Jiang Scholars Program (2013) of the China Ministry of Education; and The National Key Basic Research Program “973 project” (2015CB554000).
Authors’ contributions
ZL, HYW, SQC and CZZ conceived the study. ZL, CZZ, and FHL designed and coordinated the study. CZZ and FHL performed most experiments and the statistical analysis. HYC and NL performed several of the cellular experiments. ZLL, DDH, WXG and SHT provided the clinical parameters and survival information. HGW provided a critical review. CZZ and ZL drafted the manuscript. ZL, SQC and HYW provided funds and revised the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Cunzhen Zhang and Fenghua Liu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, C., Liu, F., Chen, H. et al. Bif-1 promotes tumor cell migration and metastasis via Cdc42 expression and activity. Clin Exp Metastasis 34, 11–23 (2017). https://doi.org/10.1007/s10585-016-9825-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-016-9825-7