Abstract
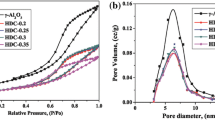
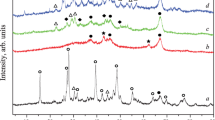
Diethyl carbonate, an important member in the family of organic carbonates, is a fuel additive like dimethyl carbonate (DMC). It holds an extra edge of having better gasoline/water distribution coefficient than DMC, and also DEC is widely used as an electrolyte in lithium ion batteries. Ethanolysis of ethyl carbamate (EC) is the most economical and greener route for DEC synthesis. Zn–Al–M (M=Ca, La, Mg and Y) have been synthesized using two methods and their activity have been explored DEC systhesis from EC and ethanol. The catalysts were characterized using thermogravimetric analysis, Brunauer, Emmett and Teller surface area, N2 adsorption–desorption textural analysis, X-ray diffraction (XRD), Fourier transform infrared spectroscopy, temperature-programmed desorption (TPD), atomic-force microscopy and Raman spectroscopy. Pure metal oxides were observed during the XRD analysis and Al2O3 was found to be in amorphous form. Third metal oxide prepared from impregnation method was found to be present on the surface as well as in impregnated form. CO2-TPD analysis showed close correlation between the basicity and the DEC yield. Zn–Al–Mg, prepared from precipitation method, being most basic, was found to be most effective although the performances of Zn–Al–Ca and Zn–Al–La were good. The effect of precipitants was also studied by synthesizing Zn–Al–Mg using NaOH and liquid NH3 as precipitants. DEC yield of 40.2% and turn over frequency of 1055 mgDEC gcat −1 h−1 was obtained in 5 h at 190 °C using Zn–Al–Mg prepared from precipitation method. Effect of reaction conditions was also studied and equilibrium constant of the reaction was estimated using the Benson group contribution method.
Graphical Abstract








Similar content being viewed by others
References
Behr A (1988) Angew Chem Int Edit 27:661–678
Zhang Y, Zhang S, Benson T (2015) Fuel Process Technol 131:7–13
Choi YJ, Sivanesan D, Lee J, Youn MH, Park KT, Kim HJ, Grace AN, Kim IH, Jeong SK (2016) J Ind Eng Chem 34:76–83
Kumar P, Srivastava VC, Gläser R, Mishra IM (2017) Powder Technol 309:13–21
Zhang Y, Zhang S, Lou HH, Gossage JL, Benson TJ (2014) Chem Eng Technol 37:1493–1499
Huang S, Liu S, Li J, Zhao N, Wei W, Sun Y (2006) Catal Lett 112:187–191
Tomishige K, Yasuda H, Yoshida Y, Nurunnabi M, Li B, Kunimori K (2004) Catal Lett 95:45–49
Eta V, Mäki-Arvela P, Salminen E, Salmi T, Murzin DY, Mikkola JP (2011) Catal Lett 141:1254
Kumar P, With P, Srivastava VC, Gläser R, Mishra IM (2017) J Alloy Compd 696:718–726
Schäffner B, Schäffner F, Verevkin SP, Börner A (2010) Chem Rev 110:4554–4581
Pacheco MA, Marshall CL (1997) Energy Fuel 11:2–29
Olmos RG, Iglesias M, Goenaga JM, Resa JM (2007) Int J Thermophys 28:1199–1227
Olmos RG, Iglesias M, Goenaga JM, Resa JM (2007) Phys Chem Liq 45:515–524
Shukla K, Srivastava VC (2017) Fuel Process Technol 161:116–124
Dunn BC, Guenneau C, Hilton SA, Pahnke J, Eyring EM, Dworzanski J, Meuzelaar HL, Hu JZ, Solum MS, Pugmire RJ (2002) Energy Fuel 16:177–181
Wang D, Yang B, Zhai X, Zhou L (2007) Fuel Process Technol 88:807–812
Zhang X, Jia D, Zhang J, Sun Y (2014) Catal Lett 144:2144–2150
Fan M, Zhang P, Ma X (2007) Fuel 86:902–905
Zhang P, Zhou Y, Fan M, Jiang P (2015) Appl Surf Sci 332:379–383
Zhang P, Zhou Y, Fan M, Jiang P (2014) Appl Surf Sci 295:50–53
Ma X, Fan M, Zhang P (2004) Catal Commun 5:765–770
Pimprom S, Sriboonkham K, Dittanet P, Föttinger K, Rupprechter G, Kongkachuichay P (2015) J Ind Eng Chem 31:156–166
Filippis PD, Scarsella M, Borgianni C, Pochetti F (2006) Energy Fuel 20:17–20
Fan M, Zhang P (2007) Energy Fuel 21:633–635
Kumar P, With P, Srivastava VC, Shukla K, Gläser R, Mishra IM (2016) RSC Adv 6:110235–110246
Keller T, Holtbruegge J, Górak A (2012) Chem Eng J 180:309–322
Shukla K, Srivastava VC (2016) RSC Adv 6:32624–32645
Shukla K, Srivastava VC (2017) Catal Rev 5:1–43
Lian G, Xinqiang Z, Hualiang A, Yanji W (2012) Chinese J Catal 33:595–600
Wang DF, Zhang XL (2012) Adv Mater Res 479:1768–1771
An H, Zhao X, Guo L, Jia C, Yuan B, Wang Y (2012) Appl Catal A 433:229–235
Qin XY, Liu B, Han B, Zhao WB, Wu SS, Lian PC (2013) Adv Mater Res 821:1081–1084
Wang L, Li H, Xin S, Li F (2014) Catal Commun 50:49–53
Xin S, Wang L, Li H, Huang K, Li F (2014) Fuel Process Technol 126:453–459
Wang P, Liu S, Zhou F, Yang B, Alshammari AS, Deng Y (2015) RSC Adv 5:19534–19540
Li F, Li H, Wang L, He P, Cao Y (2015) Catal Sci Technol 5:1021–1034
Wang D, Zhang X, Liu C, Cheng T, Wei W, Sun Y (2015) Appl Catal A 505:478–486
Zhao W, Feng D, Nong J, Cao G, Liu X, Tang Z, Chen Y (2016) React Kinet Mech Catal 117:639–654
Lee JH, Ko KH, Park BO (2003) J Cryst Growth (247:):119–125
Li J, Pan Y, Xiang C, Ge Q, Guo J (2006) Ceram Int 32:587–591
Siqingaowa Z, Yao H (2006) Front Chem China 1:277–280
ALOthman ZA (2012) Materials 5:2874–2902
Wei T, Wang M, Wei W, Sun Y, Zhong B (2003) Fuel Process Technol 83:175–182
Wang S, Zhao L, Wang W, Zhao Y, Zhang G, Ma X, Gong J (2013) Nanoscale 5:5582–5588
Rabiah-Nizah MF, Taufiq-Yap YH, Rashid U, Teo SH, Nur ZAS, Islam A (2014) Energy Convers Manag 88:1257–1262
Silambarasan M, Saravanan S, Soga T (2015) Phys E 71:109–116
Zappa D, Bertuna A, Comini E, Molinari M, Poli N, Sberveglieri G (2015) Anal Methods 7:2203–2209
Benson SW, Cruickshank FR, Golden DM, Haugen GR, O’neal HE, Rodgers AS, Shaw R, Walsh R (1969) Chem Rev 69:279–324
Zábranský M, Růžička VJ (2004) Phys Chem Ref Data 33:1071–1081
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shukla, K., Srivastava, V.C. Alkaline Earth (Ca, Mg) and Transition (La, Y) Metals Promotional Effects on Zn–Al Catalysts During Diethyl Carbonate Synthesis from Ethyl Carbamate and Ethanol. Catal Lett 147, 1891–1902 (2017). https://doi.org/10.1007/s10562-017-2097-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2097-2