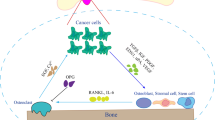
Abstract
Prostate cancer (PCa) is the most common malignancy in men. Although mortality from PCa has been declining over the past decade, metastasis can substantially shorten survival time and remains a major challenge in maintaining quality of life for survivors. PCa cells preferentially metastasize to bone and typically result in osteoblastic lesions. In the late stages of disease, however, osteolytic lesions are observed. The mechanisms of PCa bone metastasis are still unclear, but relationships between the PCa cells and the bone tissue elements are suspected of being more complex than initially thought. Far from being an innocent bystander, the bone participates actively in the metastatic process and provides the cancer cells with growth factors and a fertile environment. Among the various cells in the bone environment, osteoblasts have a central role through their bidirectional interactions with the PCa cells. This review discusses the possible mechanisms of PCa bone metastasis and highlights the essential role of osteoblasts in the metastasis of PCa to bone.
Similar content being viewed by others
References
Jemal, A., Siegel, R., Ward, E., Murray, T., Xu, J., Smigal, C., et al. (2006). Cancer statistics, 2006. CA: A Cancer Journal for Clinicians, 56, 106–130.
Cooperberg, M. R., Broering, J. M., Litwin, M. S., Lubeck, D. P., Mehta, S. S., Henning, J. M., et al. (2004). The contemporary management of prostate cancer in the United States: Lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. Journal of Urology, 171, 1393–1401.
Pienta, K. J., & Smith, D. C. (2005). Advances in prostate cancer chemotherapy: a new era begins. CA: A Cancer Journal for Clinicians, 55, 300–318; quiz 323–325.
Petrylak, D. P., Tangen, C. M., Hussain, M. H., Lara, P. N., Jr., Jones, J. A., Taplin, M. E., et al. (2004). Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. New England Journal of Medicine, 351, 1513–1520.
Galasko, C. S. (1986). Skeletal metastases. Clinical Orthopaedics and Related Research, 18–30.
Koutsilieris, M. (1993). Osteoblastic metastasis in advanced prostate cancer. Anticancer Research, 13, 443–449.
Shah, R. B., Mehra, R., Chinnaiyan, A. M., Shen, R., Ghosh, D., Zhou, M., et al. (2004). Androgen-independent prostate cancer is a heterogeneous group of diseases: Lessons from a rapid autopsy program. Cancer Research, 64, 9209–9216.
Miller, D. C., Hafez, K. S., Stewart, A., Montie, J. E., & Wei, J. T. (2003). Prostate carcinoma presentation, diagnosis, and staging: An update form the National Cancer Data Base. Cancer, 98, 1169–1178.
Bill-Axelson, A., Holmberg, L., Ruutu, M., Haggman, M., Andersson, S. O., Bratell, S., et al. (2005). Radical prostatectomy versus watchful waiting in early prostate cancer. New England Journal of Medicine, 352, 1977–1984.
Zagars, G. K., Pollack, A., & von Eschenbach, A. C. (1997). Prognostic factors for clinically localized prostate carcinoma: Analysis of 938 patients irradiated in the prostate specific antigen era. Cancer, 79, 1370–1380.
Smith, M. R., Kabbinavar, F., Saad, F., Hussain, A., Gittelman, M. C., Bilhartz, D. L., et al. (2005). Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. Journal of Clinical Oncology, 23, 2918–2925.
Chybowski, F. M., Keller, J. J., Bergstralh, E. J., & Oesterling, J. E. (1991). Predicting radionuclide bone scan findings in patients with newly diagnosed, untreated prostate cancer: Prostate specific antigen is superior to all other clinical parameters. Journal of Urology, 145, 313–318.
Mettlin, C. J., Murphy, G. P., Ho, R., & Menck, H. R. (1996). The National Cancer Data Base report on longitudinal observations on prostate cancer. Cancer, 77, 2162–2166.
Coleman, R. E. (2001). Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treatment Reviews, 27, 165–176.
Shah, J., Khaksar, S. J., & Sooriakumaran, P. (2006). Management of prostate cancer. Part 3: metastatic disease. Expert Review of Anticancer Therapy, 6, 813–821.
Cher, M. L. (2001). Mechanisms governing bone metastasis in prostate cancer. Current Opinion in Urology, 11, 483–488.
Ewing, J. (1928) (Ed.). Neoplastic diseases. Philadelphia: W.B. Saunders.
Batson, O. V. (1995). The function of the vertebral veins and their role in the spread of metastases. 1940. Clinical Orthopaedics and Related Research, 4–9.
Paget, S. (1889). The distribution of secondary growths in cancer of the breast. Lancet, 571–573.
Fidler, I. J. (2003). The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nature Reviews Cancer, 3, 453–458.
Lehr, J. E., & Pienta, K. J. (1998). Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. Journal of the National Cancer Institute, 90, 118–123.
Loberg, R. D., Fridman, Y., Pienta, B. A., Keller, E. T., McCauley, L. K., Taichman, R. S., et al. (2004). Detection and isolation of circulating tumor cells in urologic cancers: A review. Neoplasia, 6, 302–309.
Pienta, K. J., & Loberg, R. (2005). The “emigration, migration, and immigration” of prostate cancer. Clinical Prostate Cancer, 4, 24–30.
Berruti, A., Dogliotti, L., Gorzegno, G., Torta, M., Tampellini, M., Tucci, M., et al. (1999). Differential patterns of bone turnover in relation to bone pain and disease extent in bone in cancer patients with skeletal metastases. Clinical Chemistry, 45, 1240–1247.
Cook, G. B., & Watson, F. R. (1968). Events in the natural history of prostate cancer: Using salvage curves, mean age distributions and contingency coefficients. Journal of Urology, 96, 87–96.
Clarke, N. W., McClure, J., & George, N. J. (1993). Osteoblast function and osteomalacia in metastatic prostate cancer. European Urology, 24, 286–290.
Charhon, S. A., Chapuy, M. C., Delvin, E. E., Valentin-Opran, A., Edouard, C. M., & Meunier, P. J. (1983). Histomorphometric analysis of sclerotic bone metastases from prostatic carcinoma special reference to osteomalacia. Cancer, 51, 918–924.
Pecherstorfer, M., Zimmer-Roth, I., Schilling, T., Woitge, H. W., Schmidt, H., Baumgartner, G., et al. (1995). The diagnostic value of urinary pyridinium cross-links of collagen, serum total alkaline phosphatase, and urinary calcium excretion in neoplastic bone disease. Journal of Clinical Endocrinology and Metabolism, 80, 97–103.
Fontana, A., & Delmas, A. (2000). Markers of bone turnover in bone metastases. Cancer, 88, 2952–2960.
Lorente, J. A., Morote, J., Raventos, C., Encabo, G., & Valenzuela, H. (1996). Clinical efficacy of bone alkaline phosphatase and prostate specific antigen in the diagnosis of bone metastasis in prostate cancer. Journal of Urology, 155, 1348–1351.
Garnero, P., Buchs, N., Zekri, J., Rizzoli, R., Coleman, R. E., & Delmas, P. D. (2000). Markers of bone turnover for the management of patients with bone metastases from prostate cancer. British Journal of Cancer, 82, 858–864.
Zhang, J., Dai, J., Qi, Y., Lin, D. L., Smith, P., Strayhorn, C., et al. (2001). Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. Journal of Clinical Investigation, 107, 1235–1244.
Brown, J. M., Corey, E., Lee, Z. D., True, L. D., Yun, T. J., Tondravi, M., et al. (2001). Osteoprotegerin and rank ligand expression in prostate cancer. Urology, 57, 611–616.
Tu, S. M., Millikan, R. E., & Mengistu, B., et al. (2001). Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: A randomized phase II trial. The Lancet, 357, 336–341.
Turner, J. H., & Claringbold, P. G. (1991). A phase II study of treatment of painful multifocal skeletal metastases with single and repeated dose samarium-153 ethylenediaminetetramethylene phosphonate. European Journal of Cancer, 27, 1084–1086.
Collins, C., Eary, J. F., Donaldson, G., Vernon, C., Bush, N. E., Petersdorf, S., et al. (1993). Samarium-153-EDTMP in bone metastases of hormone refractory prostate carcinoma: A phase I/II trial. Journal of Nuclear Medicine, 34, 1839–1844.
Palmedo, H., Manka-Waluch, A., Albers, P., Schmidt-Wolf, I. G., Reinhardt, M., Ezziddin, S., et al. (2003). Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: Randomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. Journal of Clinical Oncology, 21, 2869–2875.
Jacobsson, H., & Naslund, I. (1991). Reduced incidence of bone metastases in irradiated areas after external radiation therapy of prostatic carcinoma. International Journal of Radiation Oncology, Biology, Physics, 20, 1297–1303.
Yin, J. J., Mohammad, K. S., Kakonen, S. M., Harris, S., Wu-Wong, J. R., Wessale, J. L., et al. (2003). A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proceedings of the National Academy of Sciences of the United States of America, 100, 10954–10959.
Nelson, J. B., Hedican, S. P., George, D. J., Reddi, A. H., Piantadosi, S., Eisenberger, M. A., et al. (1995). Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Natural Medicines, 1, 944–949.
Carducci, M. A., Padley, R. J., Breul, J., Vogelzang, N. J., Zonnenberg, B. A., Daliani, D. D., et al. (2003). Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: A randomized, phase II, placebo-controlled trial. Journal of Clinical Oncology, 21, 679–689.
Gleave, M., Hsieh, J. T., Gao, C. A., von Eschenbach, A. C., & Chung, L. W. (1991). Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Research, 51, 3753–3761.
Gleave, M. E., Hsieh, J. T., von Eschenbach, A. C., & Chung, L. W. (1992). Prostate and bone fibroblasts induce human prostate cancer growth in vivo: Implications for bidirectional tumor-stromal cell interaction in prostate carcinoma growth and metastasis. Journal of Urology, 147, 1151–1159.
Wu, H. C., Hsieh, J. T., Gleave, M. E., Brown, N. M., Pathak, S., & Chung, L. W. (1994). Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: Role of bone stromal cells. International Journal of Cancer, 57, 406–412.
Lang, S. H., Miller, W. R., & Habib, F. K. (1995). Stimulation of human prostate cancer cell lines by factors present in human osteoblast-like cells but not in bone marrow. Prostate, 27, 287–293.
Fizazi, K., Yang, J., Peleg, S., Sikes, C. R., Kreimann, E. L., Daliani, D., et al. (2003). Prostate cancer cells-osteoblast interaction shifts expression of growth/survival-related genes in prostate cancer and reduces expression of osteoprotegerin in osteoblasts. Clinical Cancer Research, 9, 2587–2597.
Rosol, T. J. (2000). Pathogenesis of bone metastases: Role of tumor-related proteins. Journal of Bone and Mineral Research, 15, 844–850.
Jacob, K., Webber, M., Benayahu, D., & Kleinman, H. K. (1999). Osteonectin promotes prostate cancer cell migration and invasion: A possible mechanism for metastasis to bone. Cancer Research, 59, 4453–4457.
Khodavirdi, A. C., Song, Z., Yang, S., Zhong, C., Wang, S., Wu, H., et al. (2006). Increased expression of osteopontin contributes to the progression of prostate cancer. Cancer Research, 66, 883–888.
Denhardt, D. T., Giachelli, C. M., & Rittling, S. R. (2001). Role of osteopontin in cellular signaling and toxicant injury. Annual Review of Pharmacology and Toxicology, 41, 723–749.
Zhang, J. H., Tang, J., Wang, J., Ma, W., Zheng, W., Yoneda, T., et al. (2003). Over-expression of bone sialoprotein enhances bone metastasis of human breast cancer cells in a mouse model. International Journal of Oncology, 23, 1043–1048.
Carmeliet, P., & Jain, R. K. (2000). Angiogenesis in cancer and other diseases. Nature, 407, 249–257.
Bianco, F. J., Jr., Powell, I. J., Cher, M. L., & Wood, D. P., Jr. (2002). Presence of circulating prostate cancer cells in African American males adversely affects survival. Urologic Oncology, 7, 147–152.
Mundy, G. R., Chen, D., Zhao, M., Dallas, S., Xu, C., & Harris, S. (2001). Growth regulatory factors and bone. Reviews in Endocrine and Metabolic Disorders, 2, 105–115.
Harris, S. E., Harris, M. A., Mahy, P., Wozney, J., Feng, J. Q., & Mundy, G. R. (1994). Expression of bone morphogenetic protein messenger RNAs by normal rat and human prostate and prostate cancer cells. Prostate, 24, 204–211.
Hamdy, F. C., Autzen, P., Robinson, M. C., Horne, C. H., Neal, D. E., & Robson, C. N. (1997). Immunolocalization and messenger RNA expression of bone morphogenetic protein-6 in human benign and malignant prostatic tissue. Cancer Research, 57, 4427–4431.
Fudge, K., Wang, C. Y., & Stearns, M. E. (1994). Immunohistochemistry analysis of platelet-derived growth factor A and B chains and platelet-derived growth factor alpha and beta receptor expression in benign prostatic hyperplasias and Gleason-graded human prostate adenocarcinomas. Modern Pathology, 7, 549–554.
Fudge, K., Bostwick, D. G., & Stearns, M. E. (1996). Platelet-derived growth factor A and B chains and the alpha and beta receptors in prostatic intraepithelial neoplasia. Prostate, 29, 282–286.
Dorkin, T. J., Robinson, M. C., Marsh, C., Neal, D. E., & Leung, H. Y. (1999). aFGF immunoreactivity in prostate cancer and its co-localization with bFGF and FGF8. Journal of Pathology, 189, 564–569.
Ferrer, F. A., Miller, L. J., Andrawis, R. I., Kurtzman, S. H., Albertsen, P. C., Laudone, V. P., et al. (1997). Vascular endothelial growth factor (VEGF) expression in human prostate cancer: in situ and in vitro expression of VEGF by human prostate cancer cells. Journal of Urology, 157, 2329–2333.
Chen, G., Shukeir, N., Potti, A., Sircar, K., Aprikian, A., Goltzman, D., et al. (2004). Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: Potential pathogenetic and prognostic implications. Cancer, 101, 1345–1356.
Koutsilieris, M., Frenette, G., Lazure, C., Lehoux, J. G., Govindan, M. V., & Polychronakos, C. (1993). Urokinase-type plasminogen activator: A paracrine factor regulating the bioavailability of IGFs in PA-III cell-induced osteoblastic metastases. Anticancer Research, 13, 481–486.
Goltzman, D. (1997). Mechanisms of the development of osteoblastic metastases. Cancer, 80, 1581–1587.
Usher, P. A., Thomsen, O. F., Iversen, P., Johnsen, M., Brunner, N., Hoyer-Hansen, G., et al. (2005). Expression of urokinase plasminogen activator, its receptor and type-1 inhibitor in malignant and benign prostate tissue. International Journal of Cancer, 113, 870–880.
Kim, I. Y., Ahn, H. J., Zelner, D. J., Shaw, J. W., Sensibar, J. A., Kim, J. H., et al. (1996). Genetic change in transforming growth factor beta (TGF-beta) receptor type I gene correlates with insensitivity to TGF-beta 1 in human prostate cancer cells. Cancer Research, 56, 44–48.
Shariat, S. F., Shalev, M., Menesses-Diaz, A., Kim, I. Y., Kattan, M. W., Wheeler, T. M., et al. (2001). Preoperative plasma levels of transforming growth factor beta(1) (TGF-beta(1)) strongly predict progression in patients undergoing radical prostatectomy. Journal of Clinical Oncology, 19, 2856–2864.
Chan, J. M., Stampfer, M. J., Giovannucci, E., Gann, P. H., Ma, J., Wilkinson, P., et al. (1998). Plasma insulin-like growth factor-I and prostate cancer risk: A prospective study. Science, 279, 563–566.
Logothetis, C. J., & Lin, S. H. (2005). Osteoblasts in prostate cancer metastasis to bone. Nature Reviews Cancer, 5, 21–28.
Vakar-Lopez, F., Cheng, C. J., Kim, J., Shi, G. G., Troncoso, P., Tu, S. M., et al. (2004). Up-regulation of MDA-BF-1, a secreted isoform of ErbB3, in metastatic prostate cancer cells and activated osteoblasts in bone marrow. Journal of Pathology, 203, 688–695.
Nielsen, O. S., Munro, A. J., & Tannock, I. F. (1991). Bone metastases: Pathophysiology and management policy. Journal of Clinical Oncology, 9, 509–524.
Clarke, N. W., McClure, J., & George, N. J. (1991). Morphometric evidence for bone resorption and replacement in prostate cancer. British Journal of Urology, 68, 74–80.
Sano, M., Kushida, K., Takahashi, M., Ohishi, T., Kawana, K., Okada, M., et al. (1994). Urinary pyridinoline and deoxypyridinoline in prostate carcinoma patients with bone metastasis. British Journal of Urology, 70, 701–703.
Takeuchi, S., Arai, K., Saitoh, H., Yoshida, K., & Miura, M. (1996). Urinary pyridinoline and deoxypyridinoline as potential markers of bone metastasis in patients with prostate cancer. Journal of Urology, 156, 1691–1695.
Roland, S. I. (1958). Calcium studies in ten cases of osteoblastic prostatic metastasis. Journal of Urology, 79, 339–342.
Thomas, R. J., Guise, T. A., Yin, J. J., Elliott, J., Horwood, N. J., Martin, T. J., et al. (1999). Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology, 140, 4451–4458.
Asadi, F., Farraj, M., Sharifi, R., Malakouti, S., Antar, S., & Kukreja, S. (1996). Enhanced expression of parathyroid hormone-related protein in prostate cancer as compared with benign prostatic hyperplasia. Human Pathology, 27, 1319–1323.
Lee, Y. P., Schwarz, E. M., Davies, M., Jo, M., Gates, J., Zhang, X., et al. (2002). Use of zoledronate to treat osteoblastic versus osteolytic lesions in a severe-combined-immunodeficient mouse model. Cancer Research, 62. 5564–5570.
Corey, E., Brown, L. G., Quinn, J. E., Poot, M., Roudier, M. P., Higano, C. S., et al. (2003). Zoledronic acid exhibits inhibitory effects on osteoblastic and osteolytic metastases of prostate cancer. Clinical Cancer Research, 9, 295–306.
Miwa, S., Mizokami, A., Keller, E. T., Taichman, R., Zhang, J., & Namiki, M. (2005). The bisphosphonate YM529 inhibits osteolytic and osteoblastic changes and CXCR-4-induced invasion in prostate cancer. Cancer Research, 65, 8818–8825.
Saad, F., Gleason, D. M., Murray, R., Tchekmedyian, S., Venner, P., Lacombe, L., et al. (2002). A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. Journal of the National Cancer Institute, 94, 1458–1468.
Saad, F., Gleason, D. M., Murray, R., Tchekmedyian, S., Venner, P., Lacombe, L., et al. (2004). Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. Journal of the National Cancer Institute, 96, 879–882.
Shahinian, V. B., Kuo, Y. F., Freeman, J. L., & Goodwin, J. S. (2005). Risk of fracture after androgen deprivation for prostate cancer. New England Journal of Medicine, 352, 154–164.
Dearnaley, D. P., Sydes, M. R., Mason, M. D., Stott, M., Powell, C. S., Robinson, A. C., et al. (2003). double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). Journal of the National Cancer Institute, 95, 1300–1311.
Gulley, J., & Dahut, W. L. (2005). Clodronate in the prevention and treatment of skeletal metastasis. Expert Review of Anticancer Therapy, 5, 221–230.
Loberg, R. D., Gayed, B. A., Olson, K. B., & Pienta, K. J. (2005). A paradigm for the treatment of prostate cancer bone metastases based on an understanding of tumor cell-microenvironment interactions. Journal of Cellular Biochemistry, 96, 439–446.
Abdollahi, A., Lipson, K. E., Han, X., Krempien, R., Trinh, T., Weber, K. J., et al. (2003). SU5416 and SU6668 attenuate the angiogenic effects of radiation-induced tumor cell growth factor production and amplify the direct anti-endothelial action of radiation in vitro. Cancer Research, 63, 3755–3763.
Jain, R. K. (2005). Antiangiogenic therapy for cancer: current and emerging concepts. Oncology (Williston Park), 19, 7–16.
Tong, R. T., Boucher, Y., Kozin, S. V., Winkler, F., Hicklin, D. J., & Jain, R. K. (2004). Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Research, 64, 3731–3736.
Kim, S. J., Uehara, H., Yazici, S., Busby, J. E., Nakamura, T., He, J., et al. (2006). Targeting platelet-derived growth factor receptor on endothelial cells of multidrug-resistant prostate cancer. Journal of the National Cancer Institute, 98, 783–793.
Thomas, A. L., Morgan, B., Horsfield, M. A., Higginson, A., Kay, A., Lee, L., et al. (2005). Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. Journal of Clinical Oncology, 23, 4162–4171.
Posey, J. A., Khazaeli, M. B., DelGrosso, A., Saleh, M. N., Lin, C. Y., Huse, W., et al. (2001). A pilot trial of Vitaxin, a humanized anti-vitronectin receptor (anti alpha v beta 3) antibody in patients with metastatic cancer. Cancer Biotherapy and Radiopharmaceuticals, 16, 125–132.
Karsenty, G. (1999). The genetic transformation of bone biology. Genes and Development, 13, 3037–3051.
Keller, E. T., Zhang, J., Cooper, C. R., Smith, P. C., McCauley, L. K., Pienta, K. J., et al. (2001). Prostate carcinoma skeletal metastases: Cross-talk between tumor and bone. Cancer and Metastasis Reviews, 20, 333–349.
Boyce, B. F., Yoneda, T., & Guise, T. A. (1999). Factors regulating the growth of metastatic cancer in bone. Endocrine Related Cancer, 6, 333–347.
Saxman, S., Ansari, R., Drasga, R., et al. (1992). Phase III trial of cyclophosphamide versus cyclophosphamide, doxorubicin, and methotrexate in hormone refractory prostatic cancer. Cancer, 70, 2488–2492.
Hudes, G., Einhorn, L., & Ross, E., et al. (1999). Vinblastine vs vinblastine plus oral estramustine phosphate for patients with hormone-refractory prostate cancer: a Hoosier Oncology Group and Fox Chase Network Phase III trial. Journal of Clinical Oncology, 17, 3160–3166.
Small, E. J., Meyer, M., Marshall, M. E., et al. (2000). Suramin therapy for patients with symptomatic hormone-refractory prostate cancer: Results of a randomized phase III trial comparing suramin plus hydrocortisone to placebo plus hydrocortisone. Journal of Clinical Oncology, 18, 1440–1450.
Kantoff, P. W., Conaway, M., & Winer, E., et al. (1999). Hydrocortisone with or without mitoxantrone in patients with hormone refractory prostate cancer: Results of the Cancer and Leukemia Group B9182 study. Journal of Clinical Oncology, 17, 2506–2513.
Tannock, I. F., Osoba, D., & Stockler, M. R., et al. (1996). Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: A Canadian randomized trial with palliative end points. Journal of Clinical Oncology, 14, 1756–1764.
Tannock, I. F., de Wit, R., Berry, W. R., Horti, J., Pluzanska, A., Chi, K. N., et al. (2004). Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New England Journal of Medicine, 351, 1502–1512.
Porter, A. T., McEwan, A. J. B., Powe, J. E., et al. (1993). Results of a randomized phase III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. International Journal of Radiation Oncology, Biology, Physics, 25, 805–813.
McCarty, M. F. (2004). Targeting multiple signaling pathways as a strategy for managing prostate cancer: multifocal signal modulation therapy. Integrative Cancer Therapies, 3, 349–380.
Uzgare, A. R., & Isaacs, J. T. (2005). Prostate cancer: Potential targets of anti-proliferative and apoptotic signaling pathways. International Journal of Biochemistry and Cell Biology, 37, 707–714.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choueiri, M.B., Tu, SM., Yu-Lee, LY. et al. The central role of osteoblasts in the metastasis of prostate cancer. Cancer Metastasis Rev 25, 601–609 (2006). https://doi.org/10.1007/s10555-006-9034-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-006-9034-y