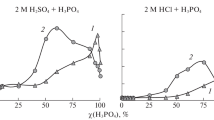
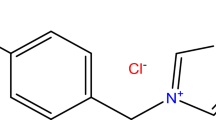
We examine the protective properties of imidazoline corrosion inhibitors with added thiourea and potassium iodide. A model of reservoir water saturated with carbon dioxide as well as hydrochloric and sulfamic acids were taken as aggressive media. The corrosion inhibition efficiency was evaluated using a gravimetric method under dynamic and static conditions in the carbon dioxide‑rich and acidic environments, respectively. The effect of individual compounds on the inhibition mechanism for metal was determined experimentally in media with different properties.




Similar content being viewed by others
References
N. K. Ishmukhamedova and A. U. Kushekov, Problems of Metal Corrosion in the Petroleum and Natural Gas Refinery Industry [in Russian], Atyrau University of Petroleum and Gas (2019), p. 9.
A. A. Igosheva, Innovatsionnaya Nauka, 11, No. 11, 71-74 (2018).
R. V. Kashkovskii and K. A. Ibatullin, Nauka i Tekhnika v Gazovoi Promyshlennosti, No. 3, 71 (2016).
L. S. Moiseeva, Zashchita Metallov, 41, No. 1, 82‑90 (2005).
A. N. Markin, Zashchita Metallov, 32, No. 5, 497 (1996).
S. Nesic, Corrosion Science, 49, 4308‑4338 (2007).
M. A. Silin et al., Acid Treatments of Reservoirs and Methods for Testing Acid Compositions [in Russian], Gubkin Russian State University of Oil and Gas, Moscow (2011), p. 120.
M. A. Silin et al., Field Chemistry [in Russian], Gubkin Russian State University of Oil and Gas, Moscow (2016).
P. A. Schweitzer, Fundamentals of Corrosion. Mechanism, Causes and Preventive Methods, CRC Press (2009), p. 426.
M. P. Chakravarthy and K. N. Mohana, International Scholarly Research Notices, 2014 (2014).
A. Edwards et al., Corrosion Science, 36, No. 2, 315‑325 (1994).
S. A. Umoren and M. M. Solomon, Journal of Industrial and Engineering Chemistry , 21, 81‑100 (2015).
S. A. Umoren, O. Ogbobe, E. E. Ebenso, et al., Pigment & Resin Technology, 35, No. 5, 284‑294 (2006).
A. V. Mamlieva, N. N. Mikhailova, and S. Yu. Savshukova, NefteGazoKhimiya, No. 1, 30‑33 (2020).
E. A. Shitikova and T. P. D’yachkova, Voprosy Sovremennoi Nauki i Praktiki, No. 3, 209‑216 (2016).
A. E. Nakulina, L. E. Odysheva, and V. S. Mal’kov, Perspectives for the Development of Basic Science. Collection of Scientific Articles of the XI International Conference of Students and Young Scientists, Tomsk April 22‑25, 2014 [in Russian], pp. 439‑441.
L. A. Magadova, K. A. Poteshkina, V. D. Vlasova, et al., Tekhnologiya Nefti i Gaza, No. 4, 14‑18 (2020).
M. A. Silin et al., Zhurnal Prikladnoi Khimii, 92, No. S13, 1732‑1741 (2019).
S. Mikhail et al., Energies, 15, No. 1, 24 (2021).
J. Tan et al., RSC Advances, 10, No. 26, 15163‑15170 (2020).
E. S. Ivanov, Inhibitors of Metal Corrosion in Acid Media [in Russian], St. Petersburg, Metallurgiya (2005).
L. Guo et al., Int. J. Electrochem. Sci., 12, No. 8, 7064‑7074 (2017).
C. B. Shen et al., Corrosion Science, 48, No. 7, 1655‑1665 (2006).
R. T. Loto et al., J. Mater. Environ. Sci., 3, No. 5, 885‑894 (2012).
D. Quy Huong, T. Duong, and P. C. Nam, ACS Omega, 4, No. 11, 14478‑14489 (2019).
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of the Russian Federation under agreement №075-15-2022-300 dated 18.04.2022 within the framework of the development program for a world-class Research Center «Efficient development of the global liquid hydrocarbon reserves».
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 6, pp. 34–39 November – December 2022
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silin, M.A., Magadova, L.A., Poteshkina, K.A. et al. Optimization of the Composition of an Imidazoline Corrosion Inhibitor for the Protection of Equipment in Carbon Dioxide-Rich and Acidic Environments. Chem Technol Fuels Oils 58, 952–956 (2023). https://doi.org/10.1007/s10553-023-01474-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-023-01474-z