Abstract
Purpose
To systematically review the literature to investigate racial disparities among bladder cancer clinical trial enrollees.
Methods
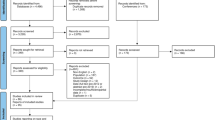
A systematic review was conducted using Ovid, MEDLINE® to identify clinical trials between 1970 and 2020. Articles were reviewed and were included if they assessed race in their outcomes reporting among bladder cancer patients enrolled in clinical trials. The review was conducted in accordance with the PRISMA statement.
Results
We identified 544 clinical trials meeting our initial search criteria, with only 24 (4.4%) studies reporting racial demographic data. Enrollees were largely Caucasian (81–98%), with a strikingly small proportion of enrolled patients consisting of African-Americans (2–8%) and Hispanics (2–5%). Only one of the studies reported results on the efficacy and safety/tolerability of the tested treatment separately for racial groups and performed analyses stratified by race.
Conclusion
Race is poorly studied in bladder cancer clinical trials. Trial cohorts may not reflect multicultural populations. The potential association between race and efficacy, safety or tolerability of the tested interventions is unknown. Given the up to twofold increase in bladder cancer-specific death among African-Americans, further research is needed to address the impact of race in clinical trials, while encompassing socioeconomic factors and disease risk factor exposures.

Similar content being viewed by others

Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to patient privacy institutional restrictions but are available from the corresponding author on reasonable request.
References
Klein JB, Nguyen CT, Saffore L, Modlin C III, Modlin CS (2010) Racial disparities in urologic health care. J Natl Med Assoc 102(2):108–118
Prout GR Jr, Wesley MN, McCarron PG et al (2004) Survival experience of black patients and white patients with bladder carcinoma. Cancer 100(3):621–630
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA 70(1):7–30
Yee DS, Ishill NM, Lowrance WT, Herr HW, Elkin EB (2011) Ethnic differences in bladder cancer survival. Urology 78(3):544–549
Cole AP, Fletcher SA, Berg S et al (2019) Impact of tumor, treatment, and access on outcomes in bladder cancer: can equal access overcome race-based differences in survival? Cancer 125(8):1319–1329
Silver JK, Bean AC, Slocum C et al (2019) Physician workforce disparities and patient care: a narrative review. Health equity 3(1):360–377
Sardar MR, Badri M, Prince CT, Seltzer J, Kowey PR (2014) Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern Med 174(11):1868–1870
Moher D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1
Higgins JP, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Guyatt G, Oxman AD, Akl EA et al (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J clin epidemiology 64(4):383–394
Lamm DL, Blumenstein BA, Crawford ED et al (1991) A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guerin for transitional-cell carcinoma of the bladder. N Engl J Med 325(17):1205–1209
Sarosdy M, deVere WR, Soloway M et al (1995) Results of a multicenter trial using the BTA test to monitor for and diagnose recurrent bladder cancer. J Urol 154(2):379–384
Smith JA Jr, Crawford ED, Paradelo JC et al (1997) Treatment of advanced bladder cancer with combined preoperative irradiation and radical cystectomy versus radical cystectomy alone: a phase III intergroup study. J Urol 157(3):805–808
Thomas L, Leyh H, Marberger M et al (1999) Multicenter trial of the quantitative BTA TRAK assay in the detection of bladder cancer. Clin Chem 45(4):472–477
Au JL-S, Badalament RA, Wientjes MG et al (2001) Methods to improve efficacy of intravesical mitomycin C: results of a randomized phase III trial. J Natl Cancer Inst 93(8):597–604
von der Maase H, Sengelov L, Roberts JT et al (2005) Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J clin oncol 23(21):4602–4608
Messing E, Kim KM, Sharkey F et al (2006) Randomized prospective phase III trial of difluoromethylornithine vs placebo in preventing recurrence of completely resected low risk superficial bladder cancer. J Urol 176(2):500–504
Sabichi AL, Lerner SP, Atkinson EN et al (2008) Phase III prevention trial of fenretinide in patients with resected non–muscle-invasive bladder cancer. Clin Cancer Res 14(1):224–229
Smith DC, Mackler NJ, Dunn RL et al (2008) Phase II trial of paclitaxel, carboplatin and gemcitabine in patients with locally advanced carcinoma of the bladder. J Urol 180(6):2384–2388
Lerner SP, Tangen CM, Sucharew H, Wood D, Crawford ED (2009) Failure to achieve a complete response to induction BCG therapy is associated with increased risk of disease worsening and death in patients with high risk non-muscle invasive bladder cancer. Urol Oncol 27(2):155–159
Sabichi AL, Lee JJ, Grossman HB et al (2011) A randomized controlled trial of celecoxib to prevent recurrence of nonmuscle-invasive bladder cancer. Cancer Prev Res 4(10):1580–1589
Messing E, Gee JR, Saltzstein DR et al (2012) A phase 2 cancer chemoprevention biomarker trial of isoflavone G-2535 (genistein) in presurgical bladder cancer patients. Cancer Prev Res 5(4):621–630
Skinner EC, Goldman B, Sakr WA et al (2013) SWOG S0353: Phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guerin. J Urol 190(4):1200–1204
Galsky MD, Hahn NM, Powles T et al (2013) Gemcitabine, cisplatin, and sunitinib for metastatic urothelial carcinoma and as preoperative therapy for muscle-invasive bladder cancer. Clin Genitourin Cancer 11(2):175–181
Lee CT, Chang SS, Kamat AM et al (2014) Alvimopan accelerates gastrointestinal recovery after radical cystectomy: a multicenter randomized placebo-controlled trial. European Urol 66(2):265–272
Hahn NM, Knudsen BS, Daneshmand S et al (2016) Neoadjuvant dasatinib for muscle-invasive bladder cancer with tissue analysis of biologic activity. Urologic oncol 34(1):4. e11-14. e17
Banerjee S, Manley K, Shaw B et al (2018) Vigorous intensity aerobic interval exercise in bladder cancer patients prior to radical cystectomy: a feasibility randomised controlled trial. Support Care Cancer 26(5):1515–1523
Gee JR, Saltzstein DR, Kim K et al (2017) A phase II randomized, double-blind, presurgical trial of Polyphenon E in bladder cancer patients to evaluate pharmacodynamics and bladder tissue biomarkers. Cancer Prev Res 10(5):298–307
Donin NM, Chamie K, Lenis AT et al (2017) A phase 2 study of TMX-101 intravesical imiquimod for the treatment of carcinoma in situ bladder cancer. Urologic oncol 35(2):39. e31-39. e37
Michaelson MD, Hu C, Pham HT et al (2017) A phase 1/2 trial of a combination of paclitaxel and trastuzumab with daily irradiation or paclitaxel alone with daily irradiation after transurethral surgery for noncystectomy candidates with muscle-invasive bladder cancer (Trial NRG Oncology RTOG 0524). Int J Radiat Oncol Biol Phys 97(5):995–1001
Messing EM, Tangen CM, Lerner SP et al (2018) Effect of intravesical instillation of gemcitabine vs saline immediately following resection of suspected low-grade non–muscle-invasive bladder cancer on tumor recurrence: SWOG S0337 randomized clinical trial. JAMA 319(18):1880–1888
Packiam VT, Lamm DL, Barocas DA et al (2018) An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non–muscle-invasive bladder cancer: Interim results. Urol Oncol 36(10):440–447
Sathianathen NJ, Fan Y, Jarosek SL, Lawrentschuk NL, Konety BR. Finasteride does not prevent bladder cancer: A secondary analysis of the Medical Therapy for Prostatic Symptoms Study. Paper presented at: Urologic Oncology: Seminars and Original Investigations2018.
Lotan Y, Inman BA, Davis LG et al (2019) Evaluation of the fluorescence in situ hybridization test to predict recurrence and/or progression of disease after Bacillus Calmette-Guerin for primary high grade nonmuscle invasive bladder cancer: results from a prospective multicenter trial. J Urol 202(5):920–926
Paul K, Sathianathen N, Dahm P, Le C, Konety BR (2019) Variation in accrual and race/ethnicity reporting in urological and nonurological related cancer trials. J Urol 202(2):385–391
Geller SE, Koch A, Pellettieri B, Carnes M (2011) Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: have we made progress? J Women’s Health 20(3):315–320
Murthy VH, Krumholz HM, Gross CP (2004) Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 291(22):2720–2726
Loree JM, Anand S, Dasari A et al (2019) Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol 5(10):e191870–e191870
Kaldany A, Blum KA, Paulucci DJ et al (2018) An evaluation of race, ethnicity, age, and sex-based representation in phase I to II renal cell carcinoma clinical trials in the United States. Urologic oncol 36(8):363. e361-363. e366
Balakrishnan AS, Palmer NR, Fergus KB et al (2019) Minority recruitment trends in phase iii prostate cancer clinical trials (2003 to 2014): progress and critical areas for improvement. J Urol 201(2):259–267
Albain KS, Unger JM, Crowley JJ, Coltman CA, Hershman DL (2009) Racial disparities in cancer suval among ranrvidomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 101(14):984–992
Eskander MF, Gil L, Beal EW et al (2022) Access denied: inequities in clinical trial enrollment for pancreatic cancer. Ann Surg Oncol 29(2):1271–1277
Rogers CR, Matthews P, Brooks E et al (2021) Barriers to and facilitators of recruitment of adult african american men for colorectal cancer research: an instrumental exploratory case study. JCO oncol pract 17(5):e686–e694
Kronenfeld JP, Graves KD, Penedo FJ, Yanez B (2021) Overcoming disparities in cancer: a need for meaningful reform for hispanic and latino cancer survivors. Oncologist 26(6):443–452
Hollenbeck BK, Dunn RL, Ye Z, Hollingsworth JM, Lee CT, Birkmeyer JD (2010) Racial differences in treatment and outcomes among patients with early stage bladder cancer. Cancer 116(1):50–56
Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD (2006) Race and surgical mortality in the United States. Ann Surg 243(2):281
Owens-Walton J, Williams C, Rompré-Brodeur A, Pinto PA, Ball MW (2022) Minority Enrollment in Phase II and III Clinical Trials in Urologic Oncology. J clin oncol. https://doi.org/10.1200/JCO.21.01885
Freudenburg E, Shan Y, Martinez A et al (2022) Geographic distribution of racial differences in mortality in muscle-invasive bladder cancer patients: an opportunity for improvement. Cancer causes control 33(4):613–622
Fletcher SA, Bivalacqua TJ, Brawley OW, Kates M (2021) Race, ethnicity, and gender reporting in North American clinical trials for BCG-unresponsive non-muscle invasive bladder cancer. Urologic oncol. https://doi.org/10.1016/j.urolonc.2021.11.015
Dess RT, Hartman HE, Mahal BA et al (2019) Association of black race with prostate cancer–specific and other-cause mortality. JAMA Oncol 5(7):975–983
Haiman CA, Stram DO, Wilkens LR et al (2006) Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med 354(4):333–342
Sirugo G, Williams SM, Tishkoff SA (2019) The missing diversity in human genetic studies. Cell 177(1):26–31
Rader DJ, Damrauer SM (2016) “Pheno”menal value for human health. Science 354(6319):1534–1536
Winkfield KM, Flowers CR, Patel JD et al (2017) American society of clinical oncology strategic plan for increasing racial and ethnic diversity in the oncology workforce. J clin oncol 35(22):2576–2579
Pirl WF, Saez-Flores E, Schlumbrecht M, Nipp R, Traeger LN, Kobetz E (2018) Race and ethnicity in the evidence for integrating palliative care into oncology. J oncol pract 14(6):e346–e356
Mitchell KA, Williams H (2019) Emerging genomic biomarkers for improving kidney, prostate, and bladder cancer health disparities outcomes. Urol Oncol. https://doi.org/10.1016/j.urolonc.2019.04.024.AccessedJun22
Funding
This study was conducted with the support of a Department of Defense Peer Reviewed Cancer Research Program (PRCRP) Career Development Award (W81XWH1710576) (SBW). Dr. Stephen B. Williams had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We would also like to thank Julie M. Trumble and Stephen Schuenke for their efforts in preparing this manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Freudenburg, E., Bagheri, I., Srinivas, S. et al. Race reporting and disparities regarding clinical trials in bladder cancer: a systematic review. Cancer Causes Control 33, 1071–1081 (2022). https://doi.org/10.1007/s10552-022-01593-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-022-01593-8