Abstract
Purpose
Breast cancer is the most common cancer and leading cause of cancer death in women. Body composition parameters, especially those related to muscle, have become a growing focus of cancer research. In this review, we summarize the literature on breast cancer and muscle parameters as well as combine their outcomes for overall survival (OS), time to tumor progression (TTP), and chemotherapy toxicity in a meta-analysis.
Methods
A systematic search of the literature for randomized controlled trials and observational studies was conducted on MEDLINE, Cochrane CENTRAL, and EMBASE through May 1, 2019. Two reviewers independently searched and selected. Meta-analysis was conducted using a random-effects model. The risk of bias was evaluated using the Newcastle–Ottawa quality assessment for cohorts and GRADE summary of findings tool from Cochrane.
Results
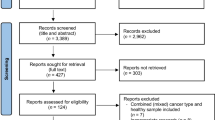
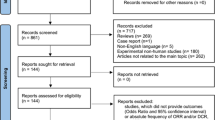
A total of 754 articles were screened from which 6 articles and one abstract were selected. Using skeletal muscle index (SMI), patients classified as sarcopenic had a 68% greater mortality risk compared to non-sarcopenic patients (HR 1.68 95% CI 1.09–2.59, 5 studies) (p = .02) (i2 = 70%). Low muscle density was not predictive of OS (HR 1.44 95% CI 0.77–2.68, 2 studies) (p = .25) (i2 = 87%). Patients with sarcopenia (56%) had more grade 3–5 toxicity compared to non-sarcopenic (25%) (RR 2.17 95% CI 1.4–3.34, 3 studies) (p = .0005) (i2 = 0%). TTP was nearly 71 days longer in advanced/metastatic patients classified as non-sarcopenic compared to patients with sarcopenia (MD − 70.75 95% CI − 122.32 to − 19.18) (p = .007) (i2 = 0%).
Conclusion
Our synthesis of the literature shows that patients with sarcopenia have more severe chemotherapy toxicity as well as shorter OS and TTP, and that low muscle density is prognostic of OS for women with metastatic breast cancer. Our findings suggest that in clinical practice, body composition assessment is valuable as a prognostic parameter in breast cancer.





Similar content being viewed by others
Abbreviations
- SMI:
-
Skeletal muscle index
- SMD:
-
Skeletal muscle density
- SMG:
-
Skeletal muscle gauge
- VAT:
-
Visceral adipose tissue
- SAT:
-
Subcutaneous adipose tissue
- CT:
-
Computer tomography
- MRI:
-
Magnetic resonance imaging
- DEXA:
-
Dual X-ray absorptiometry
- BIA:
-
Bioelectrical impedance analysis
References
WHO cancer data (2018). https://www.who.int/news-room/fact-sheets/detail/cancer
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
Torre LA, Siegel RL, Ward EM et al (2016) Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomark Prev 25:16–27
Veronesi U, Marubini E, Del Vecchio M et al (1995) Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst 87:19–27
Gobbini E, Ezzalfani M, Dieras V et al (2018) Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer 96:17–24
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127:990S–991S
Williams GR, Rier HN, McDonald A et al (2019) Sarcopenia & aging in cancer. J Geriatr Oncol 10:374–377
Mintziras I, Miligkos M, Wachter S et al (2018) Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: systematic review and meta-analysis. Int J Surg 59:19–26
Boshier PR, Heneghan R, Markar SR et al (2018) Assessment of body composition and sarcopenia in patients with esophageal cancer: a systematic review and meta-analysis. Dis Esophagus 1:1. https://doi.org/10.1093/dote/doy047
Kamarajah SK, Bundred J, Tan BHL (2019) Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer 22:10–22
Sun G, Li Y, Peng Y et al (2018) Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis 33:1419–1427
Deng HY, Hou L, Zha P et al (2019) Sarcopenia is an independent unfavorable prognostic factor of non-small cell lung cancer after surgical resection: a comprehensive systematic review and meta-analysis. Eur J Surg Oncol 45:728–735
Williams GR, Deal AM, Muss HB et al (2017) Skeletal muscle measures and physical function in older adults with cancer: sarcopenia or myopenia? Oncotarget 8:33658–33665
Kroenke CH, Prado CM, Meyerhardt JA et al (2018) Muscle radiodensity and mortality in patients with colorectal cancer. Cancer 124:3008–3015
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31:1539–1547
Akahori T, Sho M, Kinoshita S et al (2015) prognostic significance of muscle attenuation in pancreatic cancer patients treated with neoadjuvant chemoradiotherapy. World J Surg 39:2975–2982
Aust S, Knogler T, Pils D et al (2015) Skeletal muscle depletion and markers for cancer Cachexia are strong prognostic factors in epithelial ovarian cancer. PLoS ONE 10:e0140403
Centre for Reviews and Dissemination (CRD): PROSPERO: internation prospective register for systematic reviews, University of York, UK, 2019
Schardt C, Adams MB, Owens T et al (2007) Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 7:16
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Polus S, Pieper D, Burns J et al (2017) Heterogeneity in application, design, and analysis characteristics was found for controlled before-after and interrupted time series studies included in Cochrane reviews. J Clin Epidemiol 91:56–69
Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Margulis AV, Pladevall M, Riera-Guardia N et al (2014) Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol 6:359–368
von Elm E, Altman DG, Egger M et al (2014) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 12:1495–1499
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Blauwhoff-Buskermolen S, Langius JAE, Becker A et al (2017) The influence of different muscle mass measurements on the diagnosis of cancer cachexia. J Cachexia Sarcopenia Muscle 8:615–622
Del Fabbro E, Parsons H, Warneke CL et al (2012) The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist 17:1240–1245
Mazzuca F, Onesti CE, Roberto M et al (2018) Lean body mass wasting and toxicity in early breast cancer patients receiving anthracyclines. Oncotarget 9:25714–25722
Shachar SS, Deal AM, Weinberg M et al (2017) Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res 23:658–665
Sheean P, Gomez-Perez S, Joyce C et al (2019) Body composition, serum biomarkers of inflammation and quality of life in clinically stable women with estrogen receptor positive metastatic breast cancer. Nutr Cancer 71:981–991
Song EJ, Lee CW, Jung SY et al (2018) Prognostic impact of skeletal muscle volume derived from cross-sectional computed tomography images in breast cancer. Breast Cancer Res Treat 172:425–436
Versteeg KS, Blauwhoff-Buskermolen S, Buffart LM et al (2018) Higher muscle strength is associated with prolonged survival in older patients with advanced cancer. Oncologist 23:580–585
Weinberg MS, Shachar SS, Muss HB et al (2018) Beyond sarcopenia: characterization and integration of skeletal muscle quantity and radiodensity in a curable breast cancer population. Breast J 24:278–284
Rossi F, Valdora F, Barabino E et al (2019) Muscle mass estimation on breast magnetic resonance imaging in breast cancer patients: comparison between psoas muscle area on computer tomography and pectoralis muscle area on MRI. Eur Radiol 29:494–500
Caan BJ, Cespedes Feliciano EM, Prado CM et al (2018) Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol 4:798–804
Deluche E, Leobon S, Desport JC et al (2018) Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer 26:861–868
Rier HN, Jager A, Sleijfer S et al (2017) Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast 31:9–15
Villasenor A, Ballard-Barbash R, Baumgartner K et al (2012) Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv 6:398–406
Prado CM, Baracos VE, McCargar LJ et al (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926
Lee HJ, Kim HS, Kim WY et al (2019) Sarcopena as a predicting factors for chemotherapy in advanced breast cancer patients. Eur J Surg Oncol 45:e31
Rossi FVF, Bignotti B, Torri L, Succio G, Tagniafico AS (2019) Evaluation of body Computer Tomography-determined sarcopenia in breast cancer patients and clinical outcomes: a systematic review. Cancer Treatment Res Commun 21:100154
Dabiri S, Popuri K, Cespedes Feliciano EM et al (2019) Muscle segmentation in axial computed tomography (CT) images at the lumbar (L3) and thoracic (T4) levels for body composition analysis. Comput Med Imaging Graph 75:47–55
Popuri K, Cobzas D, Esfandiari N et al (2016) Body composition assessment in axial CT images using FEM-based automatic segmentation of skeletal muscle. IEEE Trans Med Imaging 35:512–520
Shachar SS, Deal AM, Weinberg M et al (2017) Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for early-stage breast cancer. Clin Cancer Res 23:3537–3543
Gonzalez MC, Heymsfield SB (2017) Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: what are we really estimating? J Cachexia Sarcopenia Muscle 8:187–189
Goodpaster BH, Thaete FL, Simoneau JA et al (1997) Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46:1579–1585
Miljkovic I, Cauley JA, Wang PY et al (2013) Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity 21:2118–2125
Feliciano E.C. LV, Chen W, Prado C., Shachar SS, Alexeeff S, Caan BJ: adiposity, muscle mass and delays and dose reduction on adjuvant taxane based chemotherapy for breast cancer. AACR2019 abstract:3284, 2019
Banerji MA, Faridi N, Atluri R et al (1999) Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab 84:137–144
Goulart A, Malheiro N, Rios H et al (2019) Influence of visceral fat in the outcomes of colorectal cancer. Dig Surg 36:33–40
Guiu B, Petit JM, Bonnetain F et al (2010) Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut 59:341–347
Ebadi M, Martin L, Ghosh S et al (2017) Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer 117:148–155
Bradshaw PT, Cespedes Feliciano EM, Prado CM et al (2019) Adipose tissue distribution and survival among women with nonmetastatic breast cancer. Obesity 27:997–1004
Hopanci Bicakli D, Cehreli R, Ozveren A et al (2019) Evaluation of sarcopenia, sarcopenic obesity, and phase angle in geriatric gastrointestinal cancer patients: before and after chemotherapy. Turk J Med Sci 49:583–588
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: european consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing 39:412–423
Chen LK, Liu LK, Woo J et al (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15:95–101
Acknowledgements
We thank for the help and guidance on our search from the University of North Carolina Health Sciences Library.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This study did not entail direct contact with humans and therefore did not entail obtaining informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aleixo, G.F.P., Williams, G.R., Nyrop, K.A. et al. Muscle composition and outcomes in patients with breast cancer: meta-analysis and systematic review. Breast Cancer Res Treat 177, 569–579 (2019). https://doi.org/10.1007/s10549-019-05352-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05352-3