Abstract
Purpose
This pilot study evaluated adherence to anti-estrogen therapy in women with hormone receptor-positive breast cancer utilizing bubble packaging.
Methods
This was a single-arm prospective investigational pilot study that enrolled 86 patients between August 2012 and April 2014. Descriptive statistics for patient age, race, insurance, stage, duration of treatment, and comorbidities were computed. All patients received routine prescriptions in a “bubble” pack or daily blister pack dispensed by one pharmacy. Participants were considered adherent if they had taken ≥ 80% of the dispensed drug. Disease-free survival (DFS) and overall survival (OS) data were obtained at 78 months.
Results
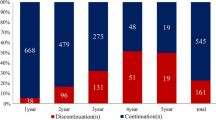
Fifty patients were included in the analysis. The overall adherence rate was 97%. None of the variables examined (race, age, insurance status, and stage) had an impact on adherence rate. Only duration of endocrine therapy had a marginal effect on adherence (p value = 0.06). The late cohort (duration of therapy 37–60 months) was least likely to be compliant at 89.53%. Our 5-year DFS was 94% and 5-year OS was 96%. There was no statistically significant difference in DFS and OS between patients with adherence rate > 90% and < 90%.
Conclusion
Adherence rate to bubble packaging was higher than that in historical studies. Although this is a single-arm pilot study, these data suggest bubble packaging of anti-estrogen may be a reasonable option to improve adherence in hormone receptor-positive breast cancer patients.

Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics. CA Cancer J Clin 68(1):7–30
Howlader N, Altekruse SF, Li CI et al (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju055
NCCN Guidelines. Breast cancer, Version 3.2018. 2018; https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed December 23, 2018
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386(10001):1341–1352
Davies C, Godwin J, Gray R et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784
Hershman DL, Shao T, Kushi LH et al (2011) Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126(2):529–537
McCowan C, Shearer J, Donnan PT et al (2008) Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 99(11):1763–1768
Partridge AH, Wang PS, Winer EP, Avorn J (2003) Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21(4):602–606
Hadji P (2010) Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol/Hematol 73(2):156–166
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99(2):215–220
Graetz I, McKillop CN, Stepanski E, Vidal GA, Anderson JN, Schwartzberg LS (2018) Use of a web-based app to improve breast cancer symptom management and adherence for aromatase inhibitors: a randomized controlled feasibility trial. J Cancer Surviv 12(4):431–440
Heiney SP, Parker PD, Felder TM, Adams SA, Omofuma OO, Hulett JM (2018) A systematic review of interventions to improve adherence to endocrine therapy. Breast Cancer Res Treat. 173(3):499–510
Conn VS, Ruppar TM, Chan KC, Dunbar-Jacob J, Pepper GA, De Geest S (2015) Packaging interventions to increase medication adherence: systematic review and meta-analysis. Curr Med Res Opin 31(1):145–160
Matthews DE, Farewell VT (2007) Using and understanding medical statistics, 4th edn. KARGER, New York
The SAS System V9 (2003) SAS Institute Inc., Cary, NC
Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R (2009) Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol 27(21):3445–3451
Lipowski EE, Campbell DE, Brushwood DB, Wilson D (1996) Time savings associated with dispensing unit-of-use packages. J Am Pharm Assoc 42(4):577–581
Heaton PC, Lin AC, Jang R, Worthen DB, Barker K (1996) Time and cost analysis of repacking medications in unit-of-use containers. J Am Pharm Assoc 40(5):631–636
Acknowledgment
This study was supported by departmental funds. Dr. Rai was supported by Wendell Cherry Chair in Clinical Trial Research. In addition, Drs. Wu and Rai were supported by the James Graham Brown Cancer Center.
Funding
This study was funded by the Division of Hematology and Medical Oncology, James Graham Brown Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhandari, S., Ngo, P., Kute, B. et al. Adherence to anti-estrogen therapy in women with hormone receptor-positive breast cancer utilizing bubble packaging: a pilot study. Breast Cancer Res Treat 177, 395–399 (2019). https://doi.org/10.1007/s10549-019-05308-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05308-7