Abstract
Purpose
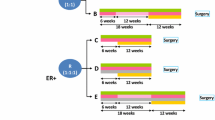
The purpose of this two-cohort Phase II trial was to estimate the pathologic complete response (pCR: ypT0/is ypN0) rate when trastuzumab plus pertuzumab are administered concurrently during both the taxane and anthracycline phases of paclitaxel and 5-fluorouracil/epirubicin/cyclophosphamide (FEC) neoadjuvant chemotherapy.
Methods
The pCR rates were assessed separately in hormone receptor (HR) positive and negative cases following Simon’s two-stage design, aiming to detect a 20% absolute improvement in pCR rates from 50 to 70 and 70 to 90% in the HR-positive and HR-`negative cohorts, respectively.
Results
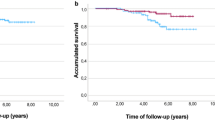
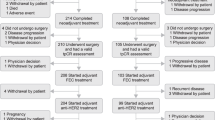
The HR-negative cohort completed full accrual of 26 patients; pCR rate was 80% (95% CI 60–91%). The HR+ cohort was closed early after 24 patients due to lower than expected pCR rate of 26% (95% CI 13–46%) at interim analysis. Overall, 44% of patients (n = 22/50) experienced grade 3/4 adverse events. The most common were neutropenia (n = 10) and diarrhea (n = 7). There was no symptomatic heart failure, but 28% (n = 14) had ≥ 10% asymptomatic decrease in LVEF; in one patient, LVEF decreased to < 50%. Cardiac functions returned to baseline by the next assessment in 57% (8/14) of cases.
Conclusions
Eighty percent of HR-negative, HER2-positive breast cancers achieve pCR with paclitaxel/FEC neoadjuvant chemotherapy administered concomitantly with pertuzumab and trastuzumab. These results are similar to pCR rates seen in trials using HER2-targeted therapy during the taxane phase only of sequential taxane–anthracycline regimens and suggest that we have reached a therapeutic plateau with HER2-targeted therapies combined with chemotherapy in the neoadjuvant setting.


Similar content being viewed by others
References
Mougalian SS, Soulos PR, Killelea BK et al (2015) Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer 121(15):2544–2552
Boughey JC, Peintinger F, Meric-Bernstam F et al (2006) Impact of preoperative versus postoperative chemotherapy on the extent and number of surgical procedures in patients treated in randomized clinical trials for breast cancer. Ann Surg 244(3):464
Symmans WF, Wei C, Gould R et al (2017) Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol 35(10):1049–1060
Buzdar AU, Valero V, Ibrahim NK et al (2007) Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2–positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res 13(1):228–233
Slamon D, Eiermann W, Robert N et al (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365(14):1273–1283
Viani GA, Afonso SL, Stefano EJ, De Fendi LI, Soares FV (2007) Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer 7(1):153
Gianni L, Eiermann W, Semiglazov V et al (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375(9712):377–384
Buzdar AU, Ibrahim NK, Francis D et al (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23(16):3676–3685
Buzdar AU, Suman VJ, Meric-Bernstam F et al (2013) Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol 14(13):1317–1325
Gianni L, Pienkowski T, Im Y-H et al (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13(1):25–32
Schneeweiss A, Chia S, Hickish T et al (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24(9):2278–2284
Baselga J, Bradbury I, Eidtmann H et al (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379(9816):633–640
Guarneri V, Frassoldati A, Bottini A et al (2012) Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2–positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol 30(16):1989–1995
Robidoux A, Tang G, Rastogi P et al (2013) Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol 14(12):1183–1192
Guarneri V, Lenihan DJ, Valero V et al (2006) Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: the MD Anderson Cancer Center experience. J Clin Oncol 24(25):4107–4115
Seidman A, Hudis C, Pierri MK et al (2002) Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20(5):1215–1221
Bayraktar S, Gonzalez-Angulo AM, Lei X et al (2012) Efficacy of neoadjuvant therapy with trastuzumab concurrent with anthracycline-and nonanthracycline-based regimens for HER2-positive breast cancer. Cancer 118(9):2385–2393
Rimawi M, Cecchini R, Rastogi P et al (2017) Abstract S3-06: A phase III trial evaluating pCR in patients with HR+, HER2-positive breast cancer treated with neoadjuvant docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) ± estrogen deprivation: NRG oncology/NSABP B-52. Cancer Res. 77(4 Supplement):S3-06–S03-06
Untch M, Loibl S, Bischoff J et al (2012) Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol 13(2):135–144
Hurvitz S, Miller J, Dichmann R et al (2013) Abstract S1-02: Final analysis of a phase II 3-arm randomized trial of neoadjuvant trastuzumab or lapatinib or the combination of trastuzumab and lapatinib, followed by six cycles of docetaxel and carboplatin with trastuzumab and/or lapatinib in patients with HER2+ breast cancer (TRIO-US B07). Cancer Res 73(24 Supplement):S1-02–S01-02
Carey LA, Berry DA, Cirrincione CT et al (2015) Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol 34(6):542–549
Hurvitz SA, Martin M, Symmans WF et al (2016) Pathologic complete response (pCR) rates after neoadjuvant trastuzumab emtansine (T-DM1 [K]) + pertuzumab (P) vs docetaxel + carboplatin + trastuzumab + P (TCHP) treatment in patients with HER2-positive (HER2+) early breast cancer (EBC) (KRISTINE). J Clin Oncol 34(suppl):500
Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA (2015) Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 150:9–16
Funding
Genentech.
Author information
Authors and Affiliations
Contributions
Study design: LP, MD, and CH. Patient accrual: SM, AS, DL, BK, AC, NH, MA-K, KS, TS, DSB, EWH, MD, and LP. Data analysis: CF, TB, JF, LR, LP, and CH. Manuscript writing: JF and LP. Final review of manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
Sarah Mougalian: Consultant/Adviser-Eisai Pharmaceuticals, Hylapharm LLC; Funding-NCCN/Pfizer, Michael DiGiovanna: Renumeration-Dako, Noemarkers (Royalties).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Foldi, J., Mougalian, S., Silber, A. et al. Single-arm, neoadjuvant, phase II trial of pertuzumab and trastuzumab administered concomitantly with weekly paclitaxel followed by 5-fluoruracil, epirubicin, and cyclophosphamide (FEC) for stage I–III HER2-positive breast cancer. Breast Cancer Res Treat 169, 333–340 (2018). https://doi.org/10.1007/s10549-017-4653-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4653-2