Abstract
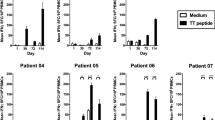
Mammaglobin-A (Mam-A) is a 10 kDa secretory protein that is overexpressed in 80 % of primary and metastatic human breast cancers. Previous studies from our laboratory demonstrated that Mam-A cDNA vaccine can induce Mam-A-specific CD8 T cell responses and mediate regression of human breast cancer xenografts in NOD/SCID mice. In this article, we present our results on a phase I clinical trial of a Mam-A cDNA vaccination in breast cancer patients with stage-IV metastatic disease, including the impact of vaccination on the expression of the inducible co-stimulator molecule (ICOS) on CD4 T cells. Specimens from seven patients with stage-IV metastatic cancer were available for these analyses. Patients were vaccinated with a Mam-A cDNA vaccine on days 0, 28, and 56, and immune responses were assessed at serial time points following vaccination. At 6 months following the first vaccination, flow cytometric analysis demonstrated a significant increase in the frequency of CD4+ICOShi T cells from 5 ± 2 % pre-vaccination to 23 ± 4 % (p < 0.001), with a concomitant decrease in the frequency of CD4+FoxP3+ T cells (regulatory T cells [Treg]) from 19 ± 6 to 10 ± 5 % (p < 0.05). ELISpot analysis of CD4+ICOShi sorted T cells demonstrated that following vaccination the cytokines produced by Mam-A-specific T cells switched from IL-10 (78 ± 21 spm pre-vaccination to 32 ± 14 spm 5 months post-vaccine p < 0.001) to IFN-γ (12 ± 6 spm pre-vaccination to 124 ± 31 spm 5 months post-vaccine p < 0.001). The ratio of CD4+ICOShi T cells to CD4+FoxP3+ T cells increased from 0.37 ± 0.12 before vaccination to 2.3 ± 0.72 (p = 0.021) following vaccination. Further, these activated CD4+ICOShi T cells induced preferential lysis of human breast cancer cells expressing Mam-A protein. We conclude that Mam-A cDNA vaccination is associated with specific expansion and activation of CD4+ICOShi T cells, with a concomitant decrease in Treg frequency. These encouraging results strongly suggest that Mam-A cDNA vaccination can induce antitumor immunity in breast cancer patients.






Similar content being viewed by others
Abbreviations
- APCs:
-
Antigen presenting cells
- ICOS:
-
Inducible co-stimulatory molecule
- Mam-A:
-
Mammaglobin-A
- mAb:
-
Monoclonal antibody
- PBMCs:
-
Peripheral blood mononuclear cells
- Treg:
-
Regulatory T cells
References
Watson MA, Fleming TP (1994) Isolation of differentially expressed sequence tags from human breast cancer. Cancer Res 54(17):4598–4602
Mikhitarian K, Gillanders WE, Almeida JS, Hebert Martin R, Varela JC, Metcalf JS, Cole DJ, Mitas M (2005) An innovative microarray strategy identities informative molecular markers for the detection of micrometastatic breast cancer. Clin Cancer Res 11(10):3697–3704
Watson MA, Dintzis S, Darrow CM, Voss LE, DiPersio J, Jensen R, Fleming TP (1999) Mammaglobin expression in primary, metastatic, and occult breast cancer. Cancer Res 59(13):3028–3031
Fleming TP, Watson MA (2000) Mammaglobin, a breast-specific gene, and its utility as a marker for breast cancer. Ann N Y Acad Sci 923:78–89
Goedegebuure PS, Watson MA, Viehl CT, Fleming TP (2004) Mammaglobin-based strategies for treatment of breast cancer. Curr Cancer Drug Targets 4(6):531–542
Gillanders WE, Mikhitarian K, Hebert R, Mauldin PD, Palesch Y, Walters C, Urist MM, Mann GB, Doherty G, Herrmann VM, Hill AD, Eremin O, El-Sheemy M, Orr RK, Valle AA, Henderson MA, Dewitty RL, Sugg SL, Frykberg E, Yeh K, Bell RM, Metcalf JS, Elliott BM, Brothers T, Robison J, Mitas M, Cole DJ (2004) Molecular detection of micrometastatic breast cancer in histopathology-negative axillary lymph nodes correlates with traditional predictors of prognosis: an interim analysis of a prospective multi-institutional cohort study. Ann Surg 239(6):828–837 (discussion 837–840)
Narayanan K, Jaramillo A, Benshoff ND, Campbell LG, Fleming TP, Dietz JR, Mohanakumar T (2004) Response of established human breast tumors to vaccination with mammaglobin-A cDNA. J Natl Cancer Inst 96(18):1388–1396
van den Broek ME, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H (1996) Decreased tumor surveillance in perforin-deficient mice. J Exp Med 184(5):1781–1790
Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD (2001) IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410(6832):1107–1111
Street SE, Hayakawa Y, Zhan Y, Lew AM, MacGregor D, Jamieson AM, Diefenbach A, Yagita H, Godfrey DI, Smyth MJ (2004) Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med 199(6):879–884
Robinson HL (1999) DNA vaccines: basic mechanism and immune responses (review). Int J Mol Med 4(5):549–555
Gurunathan S, Klinman DM, Seder RA (2000) DNA vaccines: immunology, application, and optimization*. Annu Rev Immunol 18:927–974
Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA (2001) ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 409(6816):97–101
Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G (1999) T-cell co-stimulation through B7RP-1 and ICOS. Nature 402(6763):827–832
Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, Gosselin M, Owen LR, Rudd CE, Gutierrez-Ramos JC (2000) The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity 13(1):95–105
Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, Pisa P (2006) CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol 177(10):7398–7405
Chen H, Liakou CI, Kamat A, Pettaway C, Ward JF, Tang DN, Sun J, Jungbluth AA, Troncoso P, Logothetis C, Sharma P (2009) Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci USA 106(8):2729–2734
Bharat A, Benshoff N, Fleming TP, Dietz JR, Gillanders WE, Mohanakumar T (2008) Characterization of the role of CD8+ T cells in breast cancer immunity following mammaglobin-A DNA vaccination using HLA-class-I tetramers. Breast Cancer Res Treat 110(3):453–463
Ilias Basha H, Tiriveedhi V, Fleming TP, Gillanders WE, Mohanakumar T (2011) Identification of immunodominant HLA-B7-restricted CD8+ cytotoxic T cell epitopes derived from mammaglobin-A expressed on human breast cancers. Breast Cancer Res Treat 127(1):81–89
Jaramillo A, Majumder K, Manna PP, Fleming TP, Doherty G, Dipersio JF, Mohanakumar T (2002) Identification of HLA-A3-restricted CD8+ T cell epitopes derived from mammaglobin-A, a tumor-associated antigen of human breast cancer. Int J Cancer 102(5):499–506
Jaramillo A, Narayanan K, Campbell LG, Benshoff ND, Lybarger L, Hansen TH, Fleming TP, Dietz JR, Mohanakumar T (2004) Recognition of HLA-A2-restricted mammaglobin-A-derived epitopes by CD8+ cytotoxic T lymphocytes from breast cancer patients. Breast Cancer Res Treat 88(1):29–41
Bopp SK, Lettieri T (2008) Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol 8:8
Curiel TJ (2007) Regulatory T-cell development: Is Foxp3 the decider? Nat Med 13(3):250–253
Herman AE, Freeman GJ, Mathis D, Benoist C (2004) CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med 199(11):1479–1489
Vocanson M, Rozieres A, Hennino A, Poyet G, Gaillard V, Renaudineau S, Achachi A, Benetiere J, Kaiserlian D, Dubois B, Nicolas JF (2010) Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J Allergy Clin Immunol 126(2):280–289, 289, e281–e287
Andersen MH, Schrama D, Thor Straten P, Becker JC (2006) Cytotoxic T cells. J Investig Dermatol 126(1):32–41
Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA (1999) ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397(6716):263–266
McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, Ling V, Collins M, Sharpe AH, Freeman GJ (2000) Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol 165(9):5035–5040
Kopf M, Coyle AJ, Schmitz N, Barner M, Oxenius A, Gallimore A, Gutierrez-Ramos JC, Bachmann MF (2000) Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J Exp Med 192(1):53–61
Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, Logothetis C, Sharma P (2008) CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci USA 105(39):14987–14992
Gurunathan S, Wu CY, Freidag BL, Seder RA (2000) DNA vaccines: a key for inducing long-term cellular immunity. Curr Opin Immunol 12(4):442–447
Mahajan S, Cervera A, MacLeod M, Fillatreau S, Perona-Wright G, Meek S, Smith A, MacDonald A, Gray D (2007) The role of ICOS in the development of CD4 T cell help and the reactivation of memory T cells. Eur J Immunol 37(7):1796–1808
Strauss L, Bergmann C, Szczepanski MJ, Lang S, Kirkwood JM, Whiteside TL (2008) Expression of ICOS on human melanoma-infiltrating CD4+CD25highFoxp3+ T regulatory cells: implications and impact on tumor-mediated immune suppression. J Immunol 180(5):2967–2980
Susskind B, Shornick MD, Iannotti MR, Duffy B, Mehrotra PT, Siegel JP, Mohanakumar T (1996) Cytolytic effector mechanisms of human CD4+ cytotoxic T lymphocytes. Hum Immunol 45(1):64–75
Wan YY, Flavell RA (2009) How diverse—CD4 effector T cells and their functions. J Mol Cell Biol 1(1):20–36
Aslan N, Yurdaydin C, Wiegand J, Greten T, Ciner A, Meyer MF, Heiken H, Kuhlmann B, Kaiser T, Bozkaya H, Tillmann HL, Bozdayi AM, Manns MP, Wedemeyer H (2006) Cytotoxic CD4 T cells in viral hepatitis. J Viral Hepat 13(8):505–514
Quiroga MF, Pasquinelli V, Martinez GJ, Jurado JO, Zorrilla LC, Musella RM, Abbate E, Sieling PA, Garcia VE (2006) Inducible costimulator: a modulator of IFN-gamma production in human tuberculosis. J Immunol 176(10):5965–5974
Acknowledgments
The authors would like to thank Ms. Billie Glasscock for her assistance in submitting this manuscript. This project was funded by DOD/CDMRP-BCRP W81XWH-06-1-0677 (WG). TM is funded by the BJC Foundation.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiriveedhi, V., Fleming, T.P., Goedegebuure, P.S. et al. Mammaglobin-A cDNA vaccination of breast cancer patients induces antigen-specific cytotoxic CD4+ICOShi T cells. Breast Cancer Res Treat 138, 109–118 (2013). https://doi.org/10.1007/s10549-012-2110-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2110-9