Abstract
Background and aim
Mucopolysaccharidosis IH (MPS IH, Hurler syndrome) naturally leads to death within the first decade of life, primarily from cardiac and pulmonary causes. To determine how hematopoietic stem cell transplantation (HSCT) has altered mortality, we analyzed our institution’s 30-year experience of patients with MPS IH undergoing HSCT.
Methods
Using chart review and the National Death Index, we determined survival status of 134 patients (males = 69) with MPS IH transplanted between 9/16/1983 and 7/25/2013 on 12/31/2013. Analysis included descriptive statistics, Kaplan-Meier curves, and regression analysis by Cox proportional hazards model.
Results
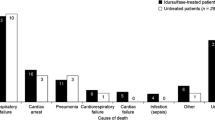
Overall survival (95% CI) at one- and 25-years was 70% (62–78%) and 37% (19–55%), respectively. From 2004 onward, overall survival at one- and 8-years was 84% (73–96%) and 81% (69–94%), respectively, compared to 65% (55–74%) and 57% (47–67%) prior to 2004 (Log-rank p = 0.032). Regardless of era, male survival was significantly better than female (HR 0.40, [95% CI: 0.21–0.74], p = 0.004). The cumulative incidence of death (95% CI) at 25 years was 63% (45–81%); incidence of pulmonary-related death was the highest at 27% (10–41%) compared to 8% (0.3–16%) for cardiac, 12% (6–17%) for infectious disease, and 16% (3–27%) from other complications.
Conclusions
HSCT has increased survival in MPS IH beyond the third decade of life and decreased the incidence of cardiac mortality, but deaths after the third year post-HSCT occur in excess of expected US mortality. It is important to determine if improved transplant strategies since 2004 result in better long-term survival in the current patient population.








Similar content being viewed by others
Abbreviations
- CDC:
-
Centers for Disease Control
- ERT:
-
enzyme replacement therapy
- GAG:
-
glycosaminoglycan
- GvHD:
-
graft-versus-host disease
- HSCT:
-
hematopoietic stem cell transplant
- IDUA:
-
alpha-L-iduronidase
- MPS IH:
-
mucopolysaccharidosis IH, Hurler syndrome
- NDI:
-
National Death Index
- TBI:
-
total body irradiation
- TLI:
-
total lymphoid irradiation
- TRM:
-
transplant-related mortality
- UCB:
-
umbilical cord blood
References
Aldenhoven M, Wynn RF, Orchard PJ, O’Meara A, Veys P, Fischer A, Valayannopoulos V et al (2015) Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: an international multicenter study. Blood 125(13):2164–2172
Ansari M, Theoret Y, Rezgui MA, Peters C, Mezziani S, Desjean C, Vachon MF et al (2014) Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematopoietic stem cell transplantation. Ther Drug Monit 36(1):93–99
Arias E (2002) United States Life Tables, 2000. Ntnl Vital Stat Rep, Centers Dis Control 51:1–39
Boelens JJ, Wynn RF, O’Meara A, Veys P, Bertrand Y, Souillet G, Wraith JE et al (2007) Outcomes of hematopoietic stem cell transplantation for Hurler’s syndrome in Europe: a risk factor analysis for graft failure. Bone Marrow Transplant 40(3):225–233
Boelens JJ, Rocha V, Aldenhoven M, Wynn R, O’Meara A, Michel G, Ionescu I et al (2009) Risk factor analysis of outcomes after unrelated cord blood transplantation in patients with Hurler syndrome. Biol Blood Marrow Transplant 15(5):618–625
Boelens JJ, Aldenhoven M, Purtill D, Ruggeri A, Defor T, Wynn R, Wraith E et al (2013) Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood 121(19):3981–3987
Centers for Disease Control (1984) Life Tables. Vital Statistics of the United States, 1980
Centers for Disease Control (1994) Life Tables. Vital Statistics of the United States, 1990
Coletti HY, Aldenhoven M, Yelin K, Poe MD, Kurtzberg J, Escolar ML (2015) Long-term functional outcomes of children with Hurler syndrome treated with unrelated umbilical cord blood transplantation. JIMD Rep 20:77–86
Cox-Brinkman J, Boelens JJ, Wraith JE, O’Meara A, Veys P, Wijburg FA, Wulffraat N et al (2006) Haematopoietic cell transplantation (HCT) in combination with enzyme replacement therapy (ERT) in patients with Hurler syndrome. Bone Marrow Transplant 38(1):17–21
Ghosh A, Miller W, Orchard PJ, Jones SA, Mercer J, Church HJ, Tylee K et al (2016) Enzyme replacement therapy prior to haematopoietic stem cell transplantation in Mucopolysaccharidosis Type I: 10 year combined experience of 2 centres. Mol Genet Metab 117(3):373–377
Guffon N, Souillet G, Maire I, Straczek J, Guibaud P (1998) Follow-up of nine patients with Hurler syndrome after bone marrow transplantation. J Pediatr 133(1):119–125
Hamidieh AA, Alimoghaddam K, Jahani M, Bahar B, Mousavi SA, Iravani M, Behfar M et al (2013) Non-TBI hematopoietic stem cell transplantation in pediatric AML patients: a single-center experience. J Pediatr Hematol Oncol 35(6):e239–e245
Hobbs JR, Hugh-Jones K, Barrett AJ, Byrom N, Chambers D, Henry K, James DC et al (1981) Reversal of clinical features of Hurler’s disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet 2(8249):709–712
Krivit W, Sung JH, Shapiro EG, Lockman LA (1995) Microglia: the effector cell for reconstitution of the central nervous system following bone marrow transplantation for lysosomal and peroxisomal storage diseases. Cell Transplant 4(4):385–392
Krovetz LJ, Lorincz AE, Schiebler GL (1965) Cardiovascular Manifestations of the Hurler Syndrome: Hemodynamic and Angiocardiographic Observations in 15 Patients. Circulation 31:132–141
Mitchell R, Nivison-Smith I, Anazodo A, Tiedemann K, Shaw PJ, Teague L, Fraser CJ et al (2013) Outcomes of haematopoietic stem cell transplantation for inherited metabolic disorders: a report from the Australian and New Zealand Children’s Haematology Oncology Group and the Australasian Bone Marrow Transplant Recipient Registry. Pediatr Transplant 17(6):582–588
Moore D, Connock MJ, Wraith E, Lavery C (2008) The prevalence of and survival in Mucopolysaccharidosis I: Hurler, Hurler-Scheie and Scheie syndromes in the UK. Orphanet J Rare Dis 3:24
Neufeld EF, Muenzer J (2001) The Mucopolysaccharidoses. The Metabolic & Molecular Bases of Inherited Disease. C. R. Scriver, A. L. Beaudet, W. S. Sly and D. Valle. New York, McGraw-Hill. III: 3421-3482.
Orchard PJ, Milla C, Braunlin E, DeFor T, Bjoraker K, Blazar BR, Peters C et al (2010) Pre-transplant risk factors affecting outcome in Hurler syndrome. Bone Marrow Transplant 45(7):1239–1246
Peters C, Balthazor M, Shapiro EG, King RJ, Kollman C, Hegland JD, Henslee-Downey J et al (1996) Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood 87(11):4894–4902
Peters C, Shapiro EG, Anderson J, Henslee-Downey PJ, Klemperer MR, Cowan MJ, Saunders EF et al (1998) Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. Storage Dis Collab Study Group Blood 91(7):2601–2608
R-Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Souillet G, Guffon N, Maire I, Pujol M, Taylor P, Sevin F, Bleyzac N et al (2003) Outcome of 27 patients with Hurler’s syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant 31(12):1105–1117
Spellman S, Setterholm M, Maiers M, Noreen H, Oudshoorn M, Fernandez-Vina M, Petersdorf E et al (2008) Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the National Marrow Donor Program Registry. Biol Blood Marrow Transplant 14(9 Suppl):37–44
Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, Allison-Thacker J et al (2004) Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med 350(19):1960–1969
Tolar J, Orchard PJ (2008) Alpha-L-iduronidase therapy for mucopolysaccharidosis type I. Biologics 2(4):743–751
Vellodi A, Young EP, Cooper A, Wraith JE, Winchester B, Meaney C, Ramaswami U et al (1997) Bone marrow transplantation for mucopolysaccharidosis type I: experience of two British centres. Arch Dis Child 76(2):92–99
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures followed were in accordance with the ethical standards of the responsible committee on human studies (institutional) and with the Helsinki Declaration of 1975, as revised in 2000.
Disclosures
Dr. Rodgers wrote the first draft of the manuscript with the assistance of his mentor, Dr. Braunlin. This study had no study sponsor. Dr. Braunlin receives research funding and travel reimbursement from BioMarin Pharmaceuticals, a company which (in conjunction with Genzyme Pharmaceutical) produces the enzyme replacement therapy discussed in this manuscript. Dr. Orchard receives research funding and travel reimbursement from both BioMarin and Genzyme. Neither BioMarin nor Genzyme played a role in the study design, the collection, analysis and interpretation or the date, the writing of the report or the decision to submit the paper for publication. Drs. Braunlin and Orchard received no financial compensation (no honorarium, grant or other form of payment) for the manuscript from either BioMarin or Genzyme.
Sources of funding
Funding came from the University of Minnesota Department of Pediatrics. Statistical support was funded in part by NCATS award UL1TR000114.
Informed consent
Informed consent was obtained from all patients or their legal guardians prior to hematopoietic stem cell transplantation for use of their medical records in research.
Animal rights
This article does not contain animal subjects.
Conflict of interest
None.
Additional information
Communicated by: Frits Wijburg
Rights and permissions
About this article
Cite this article
Rodgers, N.J., Kaizer, A.M., Miller, W.P. et al. Mortality after hematopoietic stem cell transplantation for severe mucopolysaccharidosis type I: the 30-year University of Minnesota experience. J Inherit Metab Dis 40, 271–280 (2017). https://doi.org/10.1007/s10545-016-0006-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-016-0006-2