Abstract
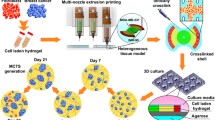
Producing three-dimensional (3-D) multicellular tumor spheroids (TSs) is valuable for characterizing anticancer drugs since they provide a more representative model of the 3-D in vivo tumor than conventional two-dimensional (2-D) monolayer culture. The interaction of tumor cells with the extracellular matrix (ECM) in a 3-D culture environment is more similar to a tumor in vivo than in a 2-D environment; cell-cell and cell-ECM interaction can influence cell behaviour, such as in response to drug treatment. In vitro tumor spheroid models have been developed using microfluidic systems to generate 3-D hydrogel beads containing components of alginate and ECM protein, such as collagen, with high uniformity and throughput. Cell-laden hydrogel droplets are formed using a flow focusing process wherein the hydrogel precursors should be a homogeneous mixture. During gelation of the droplets into beads, the alginate acts as a fast gelling component helping to maintain the spherical shape of beads and preventing coalescence as the temperature-sensitive collagen I component gels more slowly. To produce uniform hydrogel droplets using the microfluidic flow focusing system, the mixtures must be homogeneous. However, collagen’s sensitivity to temperature can lead to formation of chunks of collagen gel inside of the mixture, causing the mixture to become non-uniform and risking chip clogging. In order to overcome this limitation, previous approaches have used a cooling system during bead encapsulation while tumor cells were also present in the mixture, but this procedure can contribute to a delay in cell proliferation. Here a novel yet simple method is developed to prepare homogeneous pre-bead-encapsulation-mixtures containing collagen type I through ultrasonication. This method allows the cultivation of homogenous TS cultures with high uniformity and compact structure, and not only maintains cell viability but also the proliferation of cells in alginate/collagen hydrogel bead cultures. Depending on the sonication parameters, time and temperature, collagen can form small sized fibrils to thick fibers. Here, the mixtures containing collagen are assessed for morphology of collagen fibers/fibrils, cell viability, and proliferation. Human source Michigan Cancer Foundation-7 (MCF-7) breast cancer cells are successfully incorporated into alginate/collagen mixtures, followed by sonication, and then bead production. After bead gelation, the encapsulated MCF-7 cells remained viable and proliferated to form uniform TSs when the beads contained alginate and collagen. Results indicate that ultrasound treatment (UST) provides a powerful technique to change the structure of collagen from fiber to fibril, and to disperse collagen fibers in the mixture homogeneously for an application to generate uniform hydrogel beads and spheroids while not inhibiting cell proliferation.











Similar content being viewed by others

References
T. Armstrong et al., Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 10(21), 7427–7437 (2004). https://doi.org/10.1158/1078-0432.CCR-03-0825
C. Baylay, L. Yu, K.C. Cheung, Alginate encapsulation of cell-laden beads for microfluidic tumor spheroid culture. 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences (2013), pp. 1710–1712
Bond Energies. chemwiki.ucdavis.edu Available at: http://chemwiki.ucdavis.edu/Theoretical_Chemistry/Chemical_Bonding/General_Principles/Bond_Energies. Accessed 10 April 2015
H.F. Chan et al., Rapid formation of multicellular spheroids in double-emulsion droplets with controllable microenvironment. Sci. Rep. 3, 3462 (2013)
X. Chen, O. Nadiarynkh, S. Plotnikov, P.J. Campagnola, Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat. Protoc. 7, 654–669 (2012)
B.H. Choi, J.-I. Woo, B.-H. Min, S.R. Park, Low-intensity ultrasound stimulates the viability and matrix gene expression of human articular chondrocytes in alginate bead culture. J. Biomed. Mater. Res. A 79, 858–864 (2006)
D.L. Christiansen, E.K. Huang, F.H. Silver, Assembly of type I collagen: Fusion of fibril subunits and the influence of fibril diameter on mechanical properties. Matrix Biol. 19, 409–420 (2000)
G. Cox et al., 3-dimensional imaging of collagen using second harmonic generation. J. Struct. Biol. 141, 53–62 (2003)
F. Danhier, O. Feron, V. Préat, To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 148, 135–146 (2010)
X.M. Dong, T. Kimura, J.-F. Revol, D.G. Gray, Effects of ionic strength on the isotropic−chiral Nematic phase transition of suspensions of cellulose crystallites. Langmuir 12, 2076–2082 (1996)
P. Fratzl, Collagen: Structure and Mechanics (Springer Science & Business Media, New York, 2008)
K.A. Garvin, D.C. Hocking, D. Dalecki, Controlling the spatial organization of cells and extracellular matrix proteins in engineered tissues using ultrasound standing wave fields. Ultrasound Med. Biol. 36, 1919–1932 (2010)
K.A. Garvin, J. VanderBurgh, D.C. Hocking, D. Dalecki, Controlling collagen fiber microstructure in three-dimensional hydrogels using ultrasound. J. Acoust. Soc. Am. 134, 1491–1502 (2013)
E. Gentleman et al., Mechanical characterization of collagen fibers and scaffolds for tissue engineering. Biomaterials 24, 3805–3813 (2003)
S.M. Grist, E. Cheng, L. Yu, K.C. Cheung, Two-Photon Imaging of Whole Spheroids for Three-Dimensional Cell Cultures. 18th International Conference on Miniaturized Systems for Chemistry and Life Sciences. MicroTAS 2014 (Texas, 2014), p. 551
S.M. Grist, S.S. Nasseri, T. Poon, C. Roskelley, K.C. Cheung, On-chip clearing of arrays of 3-D cell cultures and micro-tissues. Biomicrofluidics 10, 44107 (2016a)
S.M. Grist et al., Real-Time Monitoring of Tumour Spheroid Swelling under Transient Hypoxia Using Two-Photon Microscopy. 20th Int. Conf. Miniaturized Syst. Chem. Life Sci. MicroTAS 2016 (Ireland, 2016b), p. 51
G.A. Hamilton, C. Westmoreland, E. George, Effects of medium composition on the morphology and function of rat hepatocytes cultured as spheroids and monolayers. Vitro Cell. Dev. Biol. Anim. 37, 656–667 (2001)
C.-H. Heldin, K. Rubin, K. Pietras, A. Ostman, High interstitial fluid pressure - an obstacle in cancer therapy. Nat. Rev. Cancer 4, 806–813 (2004)
W.Y. Ho, S.K. Yeap, C.L. Ho, R.A. Rahim, N.B. Alitheen, Development of multicellular tumor spheroid (MCTS) culture from breast cancer cell and a high throughput screening method using the MTT assay. PLoS One 7, e44640 (2012)
S. Hong, H.-J. Hsu, R. Kaunas, J. Kameoka, Collagen microsphere production on a chip. Lab Chip 12, 3277–3280 (2012)
Y.Y. Huang, E.M. Terentjev, Dispersion of carbon nanotubes: Mixing, sonication, stabilization, and composite properties. Polymers 4, 275–295 (2012)
D.J.S. Hulmes, Building collagen molecules, fibrils, and Suprafibrillar structures. J. Struct. Biol. 137, 2–10 (2002)
L. Huyck, C. Ampe, M. Van Troys, The XTT cell proliferation assay applied to cell layers embedded in three-dimensional matrix. Assay Drug Dev. Technol. 10, 382–392 (2012)
R.K. Jain, T. Stylianopoulos, Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010)
J.M. Kelm, N.E. Timmins, C.J. Brown, M. Fussenegger, L.K. Nielsen, Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 83, 173–180 (2003)
H.S. Khare, D.L. Burris, A quantitative method for measuring nanocomposite dispersion. Polymer 51, 719–729 (2010)
R. LaComb, O. Nadiarnykh, S.S. Townsend, P.J. Campagnola, Phase matching considerations in second harmonic generation from tissues: Effects on emission directionality, conversion efficiency and observed morphology. Opt. Commun. 281, 1823–1832 (2008)
J. Landry, D. Bernier, C. Ouellet, R. Goyette, N. Marceau, Spheroidal aggregate culture of rat liver cells: Histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J. Cell Biol. 101, 914–923 (1985)
Z.P. Luo, J.H. Koo, Quantifying the dispersion of mixture microstructures. J. Microsc. 225, 118–125 (2007)
Z.P. Luo, J.H. Koo, Quantitative study of the dispersion degree in carbon nanofiber/polymer and carbon nanotube/polymer nanocomposites. Mater. Lett. 62, 3493–3496 (2008a)
Z.P. Luo, J.H. Koo, Quantification of the layer dispersion degree in polymer layered silicate nanocomposites by transmission electron microscopy. Polymer 49, 1841–1852 (2008b)
G. Mehta, A.Y. Hsiao, M. Ingram, G.D. Luker, S. Takayama, Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 164, 192–204 (2012)
R.T. Morrison, R.N. Boyd, Organic chemistry. (Allyn and Bacon, 1966)
M. Ohi, Y. Li, Y. Cheng, T. Walz, Negative staining and image classification–powerful tools in modern electron microscopy. Biol. Proced. Online 6, 23–34 (2004)
C. Park et al., Dispersion of single wall carbon nanotubes by in situ polymerization under sonication. Chem. Phys. Lett. 364, 303–308 (2002)
G.D. Pins, D.L. Christiansen, R. Patel, F.H. Silver, Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys. J. 73, 2164–2172 (1997)
R. Ambekar Ramachandra Rao, Quantification of collagen fiber organization in biological tissues at cellular and molecular scales using second harmonic generation imaging. In: Dissertation of the Graduate College of the University of Illinois, pp. 1–149, 18 Sept 2012
R. Seemann, M. Brinkmann, T. Pfohl, S. Herminghaus, Droplet based microfluidics. Rep. Prog. Phys. 75, 16601 (2012)
W. Strober, Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. Ed. John E Coligan Al Appendix 3, Appendix 3B (2001)
M. Sun, A.B. Bloom, M.H. Zaman, Rapid quantification of 3D collagen fiber alignment and fiber intersection correlations with high sensitivity. PLoS ONE 10(7), e0131814 (2015). https://doi.org/10.1371/journal.pone.0131814
R. Sutherland, J. Carlsson, R. Durand, J. Yuhas, Spheroids in Cancer Research. Cancer Res. 41, 2980–2984 (1981)
S.-L. Tran, A. Puhar, M. Ngo-Camus, N. Ramarao, Trypan blue dye enters viable cells incubated with the pore-forming toxin HlyII of bacillus cereus. PLoS One 6(9), e22876 (2011). https://doi.org/10.1371/journal.pone.0022876
Y. Wang, J. Wang, Mixed hydrogel bead-based tumor spheroid formation and anticancer drug testing. Analyst 139, 2449–2458 (2014)
X. Wang, J.A. Kluge, G.G. Leisk, D.L. Kaplan, Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 29, 1054–1064 (2008)
M.P.E. Wenger, L. Bozec, M.A. Horton, P. Mesquida, Mechanical properties of collagen fibrils. Biophys. J. 93, 1255–1263 (2007)
R.M. Williams, W.R. Zipfel, W.W. Webb, Interpreting second-harmonic generation images of collagen I fibrils. Biophys. J. 88, 1377–1386 (2005)
Y. Yang, L.J. Kaufman, Rheology and confocal reflectance microscopy as probes of mechanical properties and structure during collagen and collagen/Hyaluronan self-assembly. Biophys. J. 96, 1566–1585 (2009)
S. Yoon, J.A. Kim, S.H. Lee, M. Kim, T.H. Park, Droplet-based microfluidic system to form and separate multicellular spheroids using magnetic nanoparticles. Lab Chip 13, 1522–1528 (2013)
L. Yu et al., Core-shell hydrogel beads with extracellular matrix for tumor spheroid formation. Biomicrofluidics 9, 24118 (2015)
J.M. Yuhas, A.P. Li, A.O. Martinez, A.J. Ladman, A simplified method for production and growth of multicellular tumor spheroids. Cancer Res. 37, 3639–3643 (1977)
Z.-J. Zhang, J. Huckle, C.A. Francomano, R.G.S. Spencer, The effects of pulsed low-intensity ultrasound on chondrocyte viability, proliferation, gene expression and matrix production. Ultrasound Med. Biol. 29, 1645–1651 (2003)
Acknowledgements
We are grateful to the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Institutes of Health Research (CIHR) for project funding. We also acknowledge The UBC Bioimaging Facility where the second harmonic generation imaging was conducted, and Derrick Horne for assistance in TEM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karamikamkar, S., Behzadfar, E. & Cheung, K.C. A novel approach to producing uniform 3-D tumor spheroid constructs using ultrasound treatment. Biomed Microdevices 20, 27 (2018). https://doi.org/10.1007/s10544-018-0260-1
Published:
DOI: https://doi.org/10.1007/s10544-018-0260-1