Abstract
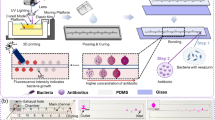
Antibiotic susceptibility testing is very important in antibiotic therapy. Traditional methods to determine antibiotic susceptibility include disk diffusion and broth dilution. However, these tests are always labor intensive, time-consuming, and need large amounts of reagents. In this paper, we demonstrated a novel pump-free micro-device that enables quantitative and high-throughput bacterial growth inhibition analysis. This device consists of a series of wells and diffusion-based antibiotic gradient channels. The wells serve as antibiotic sources and buffer sinks, and we could easily observe the bacterial growth in the gradient channels .The design of the multi-wells is adapted to the commercialized multi-channel pipette, which makes it very convenient for loading reagents into the wells. For each assay, only about 20 μL antibiotic solution is needed. As a demonstration, we used both fluorescence images and dark-field images to quantify the bacterial growth inhibition effect under different antibiotics. The quantitative data of bacterial growth inhibition under different antibiotics can be obtained within 3 to 4 h. Considering the simple operation process and the high-throughput and quantitative result this device can offer, it has great potential to be widely used in clinics and may be useful for the study of the kinetics of bacterial growth.




Similar content being viewed by others
References
J.Q. Boedicker, L. Li, T.R. Kline, R.F. Ismagilov, Lab Chip 8(8), 1265–1272 (2008)
S.Y. Cheng, S. Heilman, M. Wasserman, S. Archer, M.L. Shuler, M. Wu, Lab Chip 7(6), 763–769 (2007)
J. Choi, Y.G. Jung, J. Kim, S. Kim, Y. Jung, H. Na, S. Kwon, Lab Chip 13(2), 280–287 (2013)
J. Choi, J. Yoo, M. Lee, E.-G. Kim, J.S. Lee, S. Lee, S. Joo, S.H. Song, E.-C. Kim, J.C. Lee, H.C. Kim, Y.-G. Jung, S. Kwon, Sci. Transl. Med. 6(267), 267ra174 (2014). doi:10.1126/scitranslmed.3009650
F. Deiss, M.E. Funes-Huacca, J. Bal, K.F. Tjhung, R. Derda, Lab Chip 14(1), 167–171 (2014)
J. Fernandez, M. Navasa, J. Gomez, J. Colmenero, J. Vila, V. Arroyo, J. Rodes, Hepatology 35(1), 140–148 (2002)
J.Y. Ho, N.J. Cira, J.A. Crooks, J. Baeza, D.B. Weibel, PLoS One 7(7), e41245 (2012)
X. Jiang, Y. Kang, X. Pan, J. Yu, Q. Ouyang, C. Luo, Integr. Biol.: Quant. Biosciences Nano Macro 6(2), 143–151 (2014)
M. Kalashnikov, J.C. Lee, J. Campbell, A. Sharon, A.F. Sauer-Budge, Lab Chip 12(21), 4523–4532 (2012)
K. Lewis, Nature reviews. Drug Discovery 12(5), 371–387 (2013)
B. Li, Y. Qiu, A. Glidle, D. McIlvenna, Q. Luo, J. Cooper, H.C. Shi, H. Yin, Anal. Chem. 86(6), 3131–3137 (2014)
L. Liu, C. Luo, X. Ni, L. Wang, K. Yamauchi, S.M. Nomura, N. Nakatsuji, Y. Chen, Biomed. Microdevices 12(3), 505–511 (2010)
G. Longo, L. Alonso-Sarduy, L.M. Rio, A. Bizzini, A. Trampuz, J. Notz, G. Dietler, S. Kasas, Nat. Nanotechnol. 8(7), 522–526 (2013)
Y. Lu, J. Gao, D.D. Zhang, V. Gau, J.C. Liao, P.K. Wong, Anal. Chem. 85(8), 3971–3976 (2013)
E. Monsó, J. Ruiz, A. Rosell, J. Manterola, J. Fiz, J. Morera, V. Ausina, Am. J. Respir. Crit. Care Med. 152(4), 1316–1320 (1995)
H.C. Neu, Science 257(5073), 1064–1073 (1992)
M.R. Pulido, M. Garcia-Quintanilla, R. Martin-Pena, J.M. Cisneros, M.J. McConnell, J. Antimicrob. Chemother. 68(12), 2710–2717 (2013)
T.C. Scanlon, S.M. Dostal, K.E. Griswold, Biotechnol. Bioeng. 111(2), 232–243 (2014)
G. Si, W. Yang, S. Bi, C. Luo, Q. Ouyang, Lab Chip 12(7), 1389–1394 (2012)
D.J. Witt, D.E. Craven, W.R. McCabe, Am. J. Med. 82(5), 900–906 (1987)
Acknowledgments
We would like to thank Yang Wei, Zhaojun Li, Qi Ouyang for helpful discussions. This work is partially supported by the NSF of China (10721403, 11074009, 11174012, 11434001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ran, M., Wang, Y., Wang, S. et al. Pump-free gradient-based micro-device enables quantitative and high-throughput bacterial growth inhibition analysis. Biomed Microdevices 17, 67 (2015). https://doi.org/10.1007/s10544-015-9971-8
Published:
DOI: https://doi.org/10.1007/s10544-015-9971-8