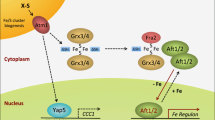
Abstract
Free radicals or reactive oxygen species (ROS) are relatively short-lived and are difficult to measure directly; so indirect methods have been explored for measuring these transient species. One technique that has been developed using Escherichia coli and Saccharomyces cerevisiae systems, relies on a connection between elevated superoxide levels and the build-up of a high-spin form of iron (Fe(III)) that is detectable by electron paramagnetic resonance (EPR) spectroscopy at g = 4.3. This form of iron is referred to as “free” iron. EPR signals at g = 4.3 are commonly encountered in biological samples owing to mononuclear high-spin (S = 5/2) Fe(III) ions in sites of low symmetry. Unincorporated iron in this study refers to this high-spin Fe(III) that is captured by desferrioxamine which is detected by EPR at g value of 4.3. Previously, we published an adaptation of Fe(III) EPR methodology that was developed for Caenorhabditis elegans, a multi-cellular organism. In the current study, we have systematically characterized various factors that modulate this unincorporated iron pool. Our results demonstrate that the unincorporated iron as monitored by Fe(III) EPR at g = 4.3 increased under conditions that were known to elevate steady-state ROS levels in vivo, including: paraquat treatment, hydrogen peroxide exposure, heat shock treatment, or exposure to higher growth temperature. Besides the exogenous inducers of oxidative stress, physiological aging, which is associated with elevated ROS and ROS-mediated macromolecular damage, also caused a build-up of this iron. In addition, increased iron availability increased the unincorporated iron pool as well as generalized oxidative stress. Overall, unincorporated iron increased under conditions of oxidative stress with no change in total iron levels. However, when total iron levels increased in vivo, an increase in both the pool of unincorporated iron and oxidative stress was observed suggesting that the status of the unincorporated iron pool is linked to oxidative stress and iron levels.






Similar content being viewed by others
References
Baylaik M, Semchyshyn H, Lushchak V (2006) Effect of hydrogen peroxide on antioxidant enzyme activities is Saccharomyces cerevisiae is strain specific. J Biochem 71:1243–1253
Bohnke R, Matzanke BF (1995) The mobile ferrous iron pool in Escherichia coli is bound to a phosphorylated sugar derivative. Biometals 8(3):223–230
Byerly L, Cassada RC, Russell RL (1976) The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction. Dev Biol 51(1):23–33. doi:10.1016/0012-1606(76)90119-6
Christen Y (2000) Oxidative stress and Alzheimer disease. Am J Clin Nutr 71(2):621S–629S
Cocheme HM, Murphy MP (2008) Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem 283(4):1786–1798
Darr D, Fridovich I (1995) Adaptation to oxidative stress in young, but not in mature or old, Caenorhabditis elegans. Free Radical Biol Med 18(2):195–201
Halliwell B, Cross CE (1994) Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect 102(Suppl 10):5–12
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press Inc., New York
Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142(2):231–255. doi:10.1038/sj.bjp.0705776
Hassan HM, Fridovich I (1978) Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem 253(22):8143–8148
Heise K, Puntarulo S, Portner HO, Abele D (2003) Production of reactive oxygen species by isolated mitochondria of the Antarctic bivalve Laternula elliptica (King and Broderip) under heat stress. Comp Biochem Physiol C: Toxicol Pharmacol 134(1):79–90
Hope IA (ed) (1999) C. elegans: a practical approach. Oxford University Press Inc., New York
Hudder BN, Morales JG, Stubna A, Munck E, Hendrich MP, Lindahl PA (2007) Electron paramagnetic resonance and Mossbauer spectroscopy of intact mitochondria from respiring Saccharomyces cerevisiae. J Biol Inorg Chem 12(7):1029–1053. doi:10.1007/s00775-007-0275-1
Jang S, Imlay JA (2007) Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J Biol Chem 282(2):929–937
Jansen WT, Bolm M, Balling R, Chhatwal GS, Schnabel R (2002) Hydrogen peroxide-mediated killing of Caenorhabditis elegans by Streptococcus pyogenes. Infect Immun 70(9):5202–5207
Jensen LT, Sanchez RJ, Srinivasan C, Valentine JS, Culotta VC (2004) Mutations in Saccharomyces cerevisiae iron-sulfur cluster assembly genes and oxidative stress relevant to Cu,Zn superoxide dismutase. J Biol Chem 279(29):29938–29943. doi:10.1074/jbc.M402795200
Keyer K, Imlay JA (1996) Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci USA 93(24):13635–13640
Kim YI, Cho JH, Yoo OJ, Ahnn J (2004) Transcriptional regulation and life-span modulation of cytosolic aconitase and ferritin genes in C.elegans. J Mol Biol 342(2):421–433
Kozlov AV, Bini A, Gallesi D, Giovannini F, Iannone A, Masini A, Meletti E, Tomasi A (1996) ‘Free’ iron, as detected by electron paramagnetic resonance spectroscopy, increases unequally in different tissues during dietary iron overload in the rat. Biometals 9(1):98–103
Levine RL, Stadtman ER (2001) Oxidative modification of proteins during aging. Exp Gerontol 36(9):1495–1502
Lin YT, Hoang H, Hsieh SI, Rangel N, Foster AL, Sampayo JN, Lithgow GJ, Srinivasan C (2006) Manganous ion supplementation accelerates wild type development, enhances stress resistance, and rescues the life span of a short-lived Caenorhabditis elegans mutant. Free Radical Biol Med 40(7):1185–1193
Link CD, Cypser JR, Johnson CJ, Johnson TE (1999) Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperones 4(4):235–242
Liu Y, Bauer SC, Imlay JA (2011) The YaaA protein of the Escherichia coli OxyR regulon lessens hydrogen peroxide toxicity by diminishing the amount of intracellular unincorporated iron. J Bacteriol 193(9):2186–2196. doi:10.1128/JB.00001-11
Melov S (2000) Mitochondrial oxidative stress. Physiologic consequences and potential for a role in aging. Ann N Y Acad Sci 908:219–225
Moy TI, Mylonakis E, Calderwood SB, Ausubel FM (2004) Cytotoxicity of hydrogen peroxide produced by Enterococcus faecium. Infect Immun 72(8):4512–4520. doi:10.1128/IAI.72.8.4512-4520.2004
Pate KT, Rangel NA, Fraser B, Clement MH, Srinivasan C (2006) Measuring “free” iron levels in Caenorhabditis elegans using low-temperature Fe(III) electron paramagnetic resonance spectroscopy. Anal Biochem 358(2):199–207
Rohrdanz E, Schmuck G, Ohler S, Kahl R (2001) The influence of oxidative stress on catalase and MnSOD gene transcription in astrocytes. Brain Res 900(1):128–136
Seaver LC, Imlay JA (2001) Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol 183(24):7182–7189. doi:10.1128/JB.183.24.7182-7189.2001
Senoo-Matsuda N, Yasuda K, Tsuda M, Ohkubo T, Yoshimura S, Nakazawa H, Hartman PS, Ishii N (2001) A defect in the cytochrome b large subunit in complex II causes both superoxide anion overproduction and abnormal energy metabolism in Caenorhabditis elegans. J Biol Chem 276(45):41553–41558. doi:10.1074/jbc.M104718200
Smith LL, Rose MS, Wyatt I (1978) The pathology and biochemistry of paraquat. Ciba Found Symp 65:321–341
Srinivasan C, Gralla EB (2002) Measurement of “free” or electron paramagnetic resonance-detectable iron in whole yeast cells as indicator of superoxide stress. Methods Enzymol 349:173–180
Srinivasan C, Liba A, Imlay JA, Valentine JS, Gralla EB (2000) Yeast lacking superoxide dismutase(s) show elevated levels of “free iron” as measured by whole cell electron paramagnetic resonance. J Biol Chem 275(38):29187–29192
Van Voorhies WA, Ward S (1999) Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. Proc Natl Acad Sci USA 96(20):11399–11403
Varghese S, Wu A, Park S, Imlay KR, Imlay JA (2007) Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli. Mol Microbiol 64(3):822–830. doi:0.1111/j.1365-2958.2007.05701.x
Wiegand C, Pehkonen S, Akkanen J, Penttinen OP, Kukkonen JV (2007) Bioaccumulation of paraquat by Lumbriculus variegatus in the presence of dissolved natural organic matter and impact on energy costs, biotransformation and antioxidative enzymes. Chemosphere 66(3):558–566
Acknowledgements
We wish to express our sincere gratitude to Dr. Gulhan Alpargu (CSU Fullerton) for statistical assistance, Drs. James Imlay (University of Illinois), Michael Bridges and Harold Rogers (CSU Fullerton) for helpful discussions, and Stephen Karl (CSU Fullerton) for his assistance with microscopy experiments. Nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. This work was partially supported by the National Institutes of Health R15 award to CS (1R15GM090169-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rangel, N.A., Lin, L., Rakariyatham, K. et al. Unincorporated iron pool is linked to oxidative stress and iron levels in Caenorhabditis elegans . Biometals 25, 971–985 (2012). https://doi.org/10.1007/s10534-012-9563-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-012-9563-5