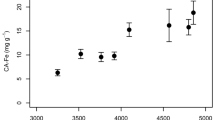
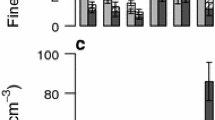
Abstract
Iron (Fe) minerals play an important role in carbon (C) and nutrient dynamics in redox fluctuating soils. We explored how the frequency of redox oscillations influence Fe reduction rates and C content in Puerto Rican soils. We hypothesized that iron reduction rates would be faster during short oscillation periods than in longer oscillation periods. Surface soils from an upland valley in a humid tropical forest were exposed to systematic redox oscillations over 49 days. The oxidation events were triggered by the introduction of air (21% O2), maintaining the time ratio under oxic or anoxic conditions at 1:6 (τox/τanox). After pre-conditioning the soil to fluctuating redox conditions for 1 month, we imposed 280- and 70-h (or 11.67- and 2.5-day) redox oscillations, measuring FeII every few days. We found that by the end of the experiment, Fe reduction rates were higher in the short oscillation period (τox = 10 h, τanox = 60 h) than in the long oscillation period (τox = 40 h, τanox = 240 h). Carbon and nitrogen loss however was similar for both treatments. These results suggest the characteristics of redox fluctuations can alter rates of Fe reduction and potentially influence ecosystem processes that depend on iron behavior.




Similar content being viewed by others
References
Aller RC (1994) Bioturbation and remineralization of sedimentary organic matter: effects of redox oscillation. Chem Geol 114:331–345
Aller RC (2004) Conceptual models of early diagenetic processes: the muddy seafloor as an unsteady, batch reactor. J Mar Res 62:815–835
Boland DD, Collins RN, Miller CJ, Glover CJ, Waite TD (2014) Effect of solution and solid-phase conditions on the Fe(II)-accelerated transformation of ferrihydrite to lepidocrocite and goethite. Environ Sci Technol 48:5477–5485
Bonneville S, Van Cappellen P, Behrends T (2004) Microbial reduction of iron (III) oxyhydroxides: effects of mineral solubility and availability. Chem Geol 212:255–268
Burdige DJ (2007) Preservation of organic matter in marine sediments: controls, mechanisms, and an imbalance in sediment organic carbon budgets? Chem Rev 107:467–485
Chacon N, Silver WL, Dubinsky EA, Cusack DF (2006) Iron reduction and soil phosphorus solubilization in humid tropical forests soils: the roles of labile carbon pools and an electron shuttle compound. Biogeochemistry 78:67–84
Cleveland CC, Wieder WR, Reed SC, Townsend AR (2010) Experimental drought in a tropical rain forest increases soil carbon dioxide losses to the atmosphere. Ecology 91:2313–2323
Coby AJ, Picardal F, Shelobolina E, Xu H, Roden EE (2011) Repeated anaerobic microbial redox cycling of iron. Appl Environ Microbiol 77:6036–6042
Colombo C, Palumbo G, He J-Z, Pinton R, Cesco S (2014) Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J Soils Sediments 14:538–548
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences and uses. Wiley, New York
Couture R-M, Charlet L, Markelova E, Bt Madé, Parsons CT (2015) On–off mobilization of contaminants in soils during redox oscillations. Environ Sci Technol 49:3015–3023
Dalgaard P (2008) Introductory statistics with R. Springer Science & Business Media, New York
Davison W, Seed G (1983) The kinetics of the oxidation of ferrous iron in synthetic and natural waters. Geochim Cosmochim Acta 47:67–79
DeAngelis KM, Silver WL, Thompson AW, Firestone MK (2010) Microbial communities acclimate to recurring changes in soil redox potential status. Environ Microbiol 12:3137–3149
Dubinsky EA, Silver WL, Firestone MK (2010) Tropical forest soil microbial communities couple iron and carbon biogeochemistry. Ecology 91:2604–2612
Echigo T, Aruguete DM, Murayama M, Hochella MF (2012) Influence of size, morphology, surface structure, and aggregation state on reductive dissolution of hematite nanoparticles with ascorbic acid. Geochim Cosmochim Acta 90:149–162
Ettwig KF, Zhu B, Speth D, Keltjens JT, Jetten MS, Kartal B (2016) Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc Natl Acad Sci USA 113:12792–12796
Eusterhues K, Rumpel C, Kögel-Knabner I (2005) Organo-mineral associations in sandy acid forest soils: importance of specific surface area, iron oxides and micropores. Eur J Soil Sci 56:753–763
Eusterhues K et al (2008) Characterization of ferrihydrite-soil organic matter coprecipitates by X-ray diffraction and Mossbauer spectroscopy. Environ Sci Technol 42:7891–7897
Favre F, Tessier D, Abdelmoula M, Genin J, Gates W, Boivin P (2002) Iron reduction and changes in cation exchange capacity in intermittently waterlogged soil. Eur J Soil Sci 53:175–183
Frierdich AJ, Helgeson M, Liu C, Wang C, Rosso KM, Scherer MM (2015) Iron atom exchange between hematite and aqueous Fe(II). Environ Sci Technol 49:8479–8486
Gimsing AL, Borggaard OK (2001) Effect of KCl and CaCl 2 as background electrolytes on the competitive adsorption of glyphosate and phosphate on goethite. Clays Clay Miner 49:270–275
Ginn BR, Habteselassie MY, Meile C, Thompson A (2014) Effects of sample storage on microbial Fe-reduction in tropical rainforest soils. Soil Biol Biochem 68:44–51
Ginn BR, Meile C, Wilmoth J, Tang Y, Thompson A (2017) Rapid iron reduction rates are stimulated by high-amplitude redox fluctuations in a tropical forest soil. Environ Sci Technol 51(6):3250–3259
Hall SJ, Silver WL (2013) Iron oxidation stimulates organic matter decomposition in humid tropical forest soils. Glob Change Biol 19:2804–2813
Hall SJ, McDowell WH, Silver WL (2013) When wet gets wetter: decoupling of moisture, redox biogeochemistry, and greenhouse gas fluxes in a humid tropical forest soil. Ecosystems 16:576–589
Hall SJ, Liptzin D, Buss HL, DeAngelis K, Silver WL (2016) Drivers and patterns of iron redox cycling from surface to bedrock in a deep tropical forest soil: a new conceptual model. Biogeochemistry 130:177–190
Hallberg KB, Grail BM, Du Plessis CA, Johnson DB (2011) Reductive dissolution of ferric iron minerals: a new approach for bio-processing nickel laterites. Miner Eng 24:620–624
Handler RM, Beard BL, Johnson CM, Scherer MM (2009) Atom exchange between aqueous Fe(II) and goethite: an Fe isotope tracer study. Environ Sci Technol 43:1102–1107
Hansen DJ, McGuire JT, Mohanty BP (2011) Enhanced biogeochemical cycling and subsequent reduction of hydraulic conductivity associated with soil-layer interfaces in the vadose zone. J Environ Qual 40:1941–1954
Hedges JI, Keil RG (1995) Sedimentary organic matter preservation: an assessment and speculative synthesis. Mar Chem 49:81–115
Henneberry YK, Kraus TE, Nico PS, Horwath WR (2012) Structural stability of coprecipitated natural organic matter and ferric iron under reducing conditions. Org Geochem 48:81–89
Hossner L et al (1996) Dissolution for total elemental analysis. In: Sparks DL et al (eds) Methods of soil analysis, Part 3-chemical methods. American Society of Agronomy, Madison, pp 49–64
Jones AM, Collins RN, Rose J, Waite TD (2009) The effect of silica and natural organic matter on the Fe(II)-catalysed transformation and reactivity of Fe(III) minerals. Geochim Cosmochim Acta 73:4409–4422
Kämpf N, Scheinost AC, Schulze DG (2011) Handbook of soil sciences: properties and processes, 2nd edn. CRC Press, Boca Raton, pp 1–34
Keiluweit M, Nico PS, Kleber M, Fendorf S (2016) Are oxygen limitations under recognized regulators of organic carbon turnover in upland soils? Biogeochemistry 127:157–171
Klueglein N, Kappler A (2013) Abiotic oxidation of Fe(II) by reactive nitrogen species in cultures of the nitrate-reducing Fe(II) oxidizer Acidovorax sp. BoFeN1–questioning the existence of enzymatic Fe(II) oxidation. Geobiology 11:180–190
Komlos J, Kukkadapu RK, Zachara JM, Jaffe PR (2007) Biostimulation of iron reduction and subsequent oxidation of sediment containing Fe-silicates and Fe-oxides: effect of redox cycling on Fe(III) bioreduction. Water Res 41:2996–3004
Konhauser KO, Kappler A, Roden EE (2011) Iron in microbial metabolisms. Elements 7:89–93
Kostka JE, Dalton DD, Skelton H, Dollhopf S, Stucki JW (2002) Growth of iron (III)-reducing bacteria on clay minerals as the sole electron acceptor and comparison of growth yields on a variety of oxidized iron forms. Appl Environ Microbiol 68:6256–6262
Lipson DA, Raab TK, Goria D, Zlamal J (2013) The contribution of Fe(III) and humic acid reduction to ecosystem respiration in drained thaw lake basins of the Arctic Coastal Plain. Global Biogeochem Cycles 27:399–409
Liptzin D, Silver WL (2009) Effects of carbon additions on iron reduction and phosphorus availability in a humid tropical forest soil. Soil Biol Biochem 41:1696–1702
Liptzin D, Silver WL, Detto M (2011) Temporal dynamics in soil oxygen and greenhouse gases in two humid tropical forests. Ecosystems 14:171–182. https://doi.org/10.1007/s10021-010-9402-x
Lovley DR (2000) Fe(III) and Mn(IV) reduction. In: Lovley DR (ed) Environmental microbe-metal interactions. American Society of Microbiology, Washington, DC, pp 3–30
Lovley DR, Phillips EJ (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480
Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory fe (iii) and mn (iv) reduction. Adv Microb Physiol 49:219–286
Marumoto T, Anderson J, Domsch K (1982) Mineralization of nutrients from soil microbial biomass. Soil Biol Biochem 14:469–475
McNicol G, Silver WL (2014) Separate effects of flooding and anaerobiosis on soil greenhouse gas emissions and redox sensitive biogeochemistry. J Geophys Res 119:557–566
Megonigal J, Mines M, Visscher P (2005) Linkages to trace gases and aerobic processes. Biogeochemistry 8:350–362
Mejia J, Roden EE, Ginder-Vogel M (2016) Influence of oxygen and nitrate on Fe (hydr) oxide mineral transformation and soil microbial communities during redox cycling. Environ Sci Technol 50:3580–3588
Melamed R, Boas RV (1998) Phosphate-background electrolyte interaction affecting the transport of mercury through a Brazilian Oxisol. Sci Total Environ 213:151–156
Mikutta R, Kleber M, Torn MS, Jahn R (2006) Stabilization of soil organic matter: association with minerals or chemical recalcitrance? Biogeochemistry 77:25–56
Muggler C, Pape T, Buurman P (1997) Laser grain-size determination in soil genetic studies 2. Clay content, clay formation, and aggregation in some Brazilian oxisols. Soil Sci 162:219–228
Novais R, Smyth T (1999) Phosphorus in soil and plant in tropical conditions. Federal University of Viçosa, Viçosa, p 399
Peretyazhko T, Sposito G (2005) Iron (III) reduction and phosphorous solubilization in humid tropical forest soils. Geochim Cosmochim Acta 69:3643–3652
Pett-Ridge J, Silver WL, Firestone MK (2006) Redox fluctuations frame microbial community impacts on N-cycling rates in a humid tropical forest soil. Biogeochemistry 81:95–110
Porras RC, Pries CEH, McFarlane KJ, Hanson PJ, Torn MS (2017) Association with pedogenic iron and aluminum: effects on soil organic carbon storage and stability in four temperate forest soils. Biogeochemistry 133(3):333–345
Reddy K, Feijtel T, Patrick W (1986) Effect of soil redox conditions on microbial oxidation of organic matter. In: Chen Y, Avnimelech Y (eds) The role of organic matter in modern agriculture. Springer, New York, pp 117–156
Roden EE, Wetzel RG (1996) Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol Oceanogr 41:1733–1748
Roden EE, Zachara JM (1996) Microbial reduction of crystalline iron (III) oxides: influence of oxide surface area and potential for cell growth. Environ Sci Technol 30:1618–1628
Scatena FN (1989) An introduction to the physiography and history of the Bisley Experimental Watersheds in the Luquillo Mountains of Puerto Rico. US Dept of Agriculture, Forest Service, Southern Forest Experiment Station, New Orleans, p 72
Schulte P et al (2016) Influence of HCl pretreatment and organo-mineral complexes on laser diffraction measurement of loess–paleosol-sequences. CATENA 137:392–405
Schuur EA, Chadwick OA, Matson PA (2001) Carbon cycling and soil carbon storage in mesic to wet Hawaiian montane forests. Ecology 82:3182–3196
Sekar R, DiChristina TJ (2014) Microbially driven Fenton reaction for degradation of the widespread environmental contaminant 1, 4-dioxane. Environ Sci Technol 48:12858–12867
Silver WL, Lugo A, Keller M (1999) Soil oxygen availability and biogeochemistry along rainfall and topographic gradients in upland wet tropical forest soils. Biogeochemistry 44:301–328
Silver WL, Herman DJ, Firestone MK (2001) Dissimilatory nitrate reduction to ammonium in upland tropical forest soils. Ecology 82:2410–2416
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404
Sposito G (2008) The chemistry of soils. Oxford University Press, Oxford
Straub KL, Benz M, Schink B, Widdel F (1996) Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl Environ Microbiol 62:1458–1460
Takai Y, Kamura T (1966) The mechanism of reduction in waterlogged paddy soil. Folia Microbiol 11:304–313
Teh YA, Dubinsky EA, Silver WL, Carlson CM (2008) Suppression of methanogenesis by dissimilatory Fe(III)-reducing bacteria in tropical rain forest soils: implications for ecosystem methane flux. Glob Change Biol 14:413–422
Thaymuang W, Kheoruenromne I, Suddhipraharn A, Sparks DL (2013) The role of mineralogy in organic matter stabilization in tropical soils. Soil Sci 178:308–315
Thomé RCM (1954) A survey of the geology of Puerto Rico. University of Puerto Rico, Agricultural Experiment Station, Puerto Rico
Thompson A, Chadwick OA, Rancourt DG, Chorover J (2006) Iron-oxide crystallinity increases during soil redox oscillations. Geochim Cosmochim Acta 70:1710–1727
Tishchenko V, Meile C, Scherer MM, Pasakarnis TS, Thompson A (2015) Fe 2 + catalyzed iron atom exchange and re-crystallization in a tropical soil. Geochim Cosmochim Acta 148:191–202
Tomaszewski EJ, Cronk SS, Gorski CA, Ginder-Vogel M (2016) The role of dissolved Fe(II) concentration in the mineralogical evolution of Fe (hydr) oxides during redox cycling. Chem Geol 438:163–170
Vogelsang V, Fiedler S, Jahn R, Kaiser K (2016) In-situ transformation of iron-bearing minerals in marshland-derived paddy subsoil. Eur J Soil Sci 67:676–685
Weber KA, Achenbach LA, Coates JD (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–764
Wilmoth JL (2016) Effects of redox-cycling on iron-mineral transformations and metatranscriptome of iron (III)-reducing bacteria in a humid tropical forest soil. Dissertation. University of Georgia
Yang J, Kukkadapu RK, Dong H, Shelobolina ES, Zhang J, Kim J (2012) Effects of redox cycling of iron in nontronite on reduction of technetium. Chem Geol 291:206–216
Yao H, Conrad R, Wassmann R, Neue H (1999) Effect of soil characteristics on sequential reduction and methane production in sixteen rice paddy soils from China, the Philippines, and Italy. Biogeochemistry 47:269–295
Yu G et al (2017) Mineral availability as a key regulator of soil carbon storage. Environ Sci Technol 51:4960–4969
Zak D, Wagner C, Payer B, Augustin J, Gelbrecht J (2010) Phosphorus mobilization in rewetted fens: the effect of altered peat properties and implications for their restoration. Ecol Appl 20:1336–1349
Zobeck TM (2004) Rapid soil particle size analyses using laser diffraction. Appl Eng Agric 20:633
Acknowledgements
Thanks for scholarship support from the CAPES Foundation, Ministry of Education of Brazil to DB. We thank Whendee Silver for providing field samples, Allan Bacon and Julio Pachon for conducting soil texture analysis, and members of the Thompson Lab (especially Jared Wilmoth) for technical assistance. We also thank two annoymous reviewers and the associate editor whose helpful comments greatly improved the manuscript.
Funding
Funding for this research was provided by National Science Foundation (NSF), Grants EAR-1331841, EAR-1331846, EAR-1451508 and DEB-1457761 to AT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Melany Fisk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barcellos, D., Cyle, K.T. & Thompson, A. Faster redox fluctuations can lead to higher iron reduction rates in humid forest soils. Biogeochemistry 137, 367–378 (2018). https://doi.org/10.1007/s10533-018-0427-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-018-0427-0