Abstract
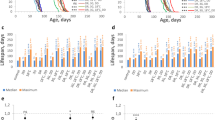
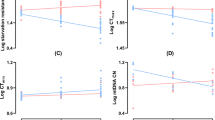
Longevity of a species is a multifactorial quantitative trait influenced by genetic background, sex, age and environment of the organism. Extended longevity phenotypes (ELP) from experimental evolution in the laboratory can be used as model systems to investigate the mechanisms underlying aging and senescence. ELPs of Drosophila are correlated with various life history attributes such as resistance to environmental stressors (starvation, desiccation, cold and paraquat), developmental time, biochemical defenses, etc. The association between oxidative stress resistance and longevity is not clear and ELPs offer an opportunity to examine the role of oxidative stress resistance in longevity. Here, we have investigated the hypothesis that enhanced oxidative stress resistance and elevated antioxidant defense system play a positive role in longevity using an ELP of Drosophila melanogaster. An ELP of D. melanogaster isolated and characterized in our laboratory through artificial selection (inbred laboratory strain of Oregon K) is employed in this study. Our ELP, named as long lifespan (LLS) flies, shows marked extension in lifespan when compared to the progenitor population (normal lifespan, NLS) and makes a suitable model to study the role of mitochondrial genome in longevity because of its least heterogeneity. In this study, sensitivity to ethanol with age was employed as a measure of resistance to oxidative stress in NLS and LLS flies. Effect of age and oxidative stress on longevity was examined by employing NLS and LLS flies of different age groups against ethanol-induced oxidative stress. Results show that the lower mortality against ethanol was associated with enhanced oxidative stress resistance, higher antioxidant defenses, lower reactive oxygen species (ROS) levels, enhanced alcohol dehydrogenase activity and better locomotor ability attributes of LLS flies. In addition, age-related changes like locomotor impairments, decreased antioxidant defenses, higher ROS levels and sensitivity to oxidative stress were delayed in LLS flies when compared to NLS. Our study supports the hypothesis that higher oxidative stress resistance and enhanced antioxidant defenses are significant factors in extending longevity.









Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 05:121–125
Archer CR, Sakaluk SK, Selman C, Royle NJ, Hunt J (2013) Oxidative stress and the evolution of sex differences in lifespan and ageing in the decorated cricket, Gryllodes sigillatus. Evolution 67:620–634
Arking R (1987) Successful selection for increased longevity in Drosophila: analysis of the survival data and presentation of a hypothesis on the genetic regulation of longevity. Exp Gerontol 22:199–220
Arking R, Buck S, Berrios A, Dwyer S, Baker GT 3rd (1991) Elevated paraquat resistance can be used as a bioassay for longevity in a genetically based long-lived strain of Drosophila. Dev Genet 12:362–370
Arking R, Burde V, Graves K, Hari R, Feldman E, Zeevi A, Soliman S, Saraiya A, Buck S, Vettraino J, Sathrasala K (2000a) Identical longevity phenotypes are characterized by different patterns of gene expression and oxidative damage. Exp Gerontol 35:353–373
Arking R, Burde V, Graves K, Hari R, Feldman E, Zeevi A, Soliman S, Saraiya A, Buck S, Vettraino J, Sathrasala K, Wehr N, Levine RL (2000b) Forward and reverse selection for longevity in Drosophila is characterized by alteration of antioxidant gene expression and oxidative damage patterns. Exp Gerontol 35:167–185
Arking R, Buck S, Hwangbo D-S, Lane M (2002) Metabolic alterations and shift in energy allocations are corequisites for the expression of extended longevity genes in Drosophila. Ann N Y Acad Sci 959:251–262
Awofala AA, Davies JA, Jones S (2012) Functional roles for redox genes in ethanol sensitivity in Drosophila. Funct Integr Genom 12:305–315
Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78:547–581
Black MJ, Brandt RB (1974) Spectrofluorometric analysis of hydrogen peroxide. Anal Biochem 1:246–254
Bubliy OA, Loeschcke V (2005) Correlated responses to selection for stress resistance and longevity in a laboratory population of Drosophila melanogaster. J Evol Biol 18:789–803
Comporti M, Signorini C, Leoncini S, Gardi C, Ciccoli L, Giardini A, Vecchio D, Arezzini B (2010) Ethanol-induced oxidative stress: basic knowledge. Genes Nutr 5:101–109
Das SK, Vasudevan DM (2007) Alcohol-induced oxidative stress. Life Sci 81:177–187
David JR, Bocquet C, Arens M-F, Fouillet P (1976) Biological role of alcohol dehydrogenase in the tolerance of Drosophila melanogaster to aliphatic alcohols: utilization of an ADH-null mutant. Biochem Genet 14:989–997
Deepashree S, Haddadi M, Ramesh SR, Shivanandappa T (2012) Isolation of a long lifespan strain of Drosophila melanogaster. Drosoph Inf Serv 95:101–103
Deepashree S, Shivanandappa T, Ramesh SR (2017) Life history traits of an extended longevity phenotype of Drosophila melanogaster. Curr Aging Sci 10:224–238
Deepashree S, Shivanandappa T, Ramesh SR (2018) Is longevity a heritable trait? evidence for non-genomic influence from an extended longevity phenotype of Drosophila melanogaster. Curr Aging Sci 11:24–32
Delcour J (1969) A rapid and efficient method of egg collecting. Drosoph Inf Serv 44:133–134
Devineni AV, McClure KD, Guarnieri DJ, Corl AB, Wolf FW, Eddison M, Heberlein U (2011) The genetic relationships between ethanol preference, acute ethanol sensitivity and ethanol tolerance in Drosophila melanogaster. Fly 5:191–199
Dudas SP, Arking R (1995) Coordinate upregulation of the antioxidant gene activities is associated with the delayed onset of senescence in a long lived strain of Drosophila. J Gerontol Biol Sci 50A:B117–B127
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of cholinesterase activity. Biochem Pharmacol 7:88–95
Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sanchez-Perez P, Cadenas S, Lamas S (2015) Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol 6:183–197
Feany MB, Bender WW (2000) A Drosophila model of Parkinson’s disease. Nature 404:394–398
Fry JD, Bahnck CM, Mikucki M, Phadnis N, Slattery WC (2004) Dietary ethanol mediates selection on aldehyde dehydrogenase activity in Drosophila melanogaster. Integr Comp Biol 44:275–283
Geer BW, McKechnie SW, Bentley MM, Oakeshott JG, Quinn EM, Langevin ML (1988) Induction of alcohol dehydrogenase by ethanol in Drosophila melanogaster. J Nutr 118:398–407
Guarnieri DJ, Heberlein U (2003) Drosophila melanogaster, a genetic model system for alcohol research. Int Rev Neurobiol 54:199–228
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300
Hercus MJ, Loeschcke V, Rattan SI (2003) Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology 4:149–156
Hoffman AA, Parsons PA (1989) Selection for increased desiccation resistance in Drosophila melanogaster: additive genetic control and correlated responses for other stresses. Genetics 122:837–845
Jahromi SR, Haddadi M, Shivanandappa T, Ramesh SR (2015) Modulatory effect of Decalepis hamiltonii on ethanol-induced toxicity in transgenic Drosophila model of Parkinson’s disease. Neurochem Int 80:1–6
Jones DP (2015) Redox theory of aging. Redox Biol 5:71–79
Kenyon C (2005) The plasticity of aging: insights from long-lived mutants. Cell 120:449–460
Kirkwood TB (1977) Evolution of ageing. Nature 270:301–304
Kirkwood TB, Kowald A (2012) The free-radical theory of ageing–older, wiser and still alive: modeling positional effects of the primary targets of ROS reveals new support. BioEssays 34:692–700
Kuether K, Arking R (1999) Drosophila selected for extended longevity are more sensitive to heat shock. Age 22:175–180
Liochev SI (2015) Which is the most significant cause of aging? Antioxidants 4:793–810
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Luckinbill LS, Arking R, Clare MJ, Cirocco WC, Buck SA (1984) Selection for delayed senescence in Drosophila melanogaster. Evolution 38:996–1003
Marklund SL, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
McKenzie JA, Parsons PA (1972) Alcohol tolerance: an ecological parameter in the relative success of Drosophila melanogaster and Drosophila simulans. Oecologia 10:373–388
Medawar PB (1952) An unsolved problem of biology. H. K. Lewis and Company, London
Mockett RJ, Orr WC, Rahmandar JJ, Sohal BH, Sohal RS (2001) Antioxidant status and stress resistance in long- and short-lived lines of Drosophila melanogaster. Exp Gerontol 36:441–463
Montooth KL, Siebenthall KT, Clark AG (2006) Membrane lipid physiology and toxin catabolism underlie ethanol and acetic acid tolerance in Drosophila melanogaster. J Exp Biol 209:3837–3850
Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U (1998) Ethanol intoxication in Drosophila: genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell 93:997–1007
Niveditha S, Deepashree S, Ramesh SR, Shivanandappa T (2017) Sex differences in oxidative stress resistance in relation to longevity in Drosophila melanogaster. J Comp Physiol B 187:899–909
Orr WC, Sohal RS (1994) Extension of life span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 263:1128–1130
Paaby AB, Schmidt PS (2009) Dissecting the genetics of longevity in Drosophila melanogaster. Fly (Austin) 3:29–38
Partridge L, Barton NH (1993) Optimality, mutation and the evolution of ageing. Nature 362:305–311
Partridge L, Fowler K (1992) Direct and correlated responses to selection on age at reproduction in Drosophila melanogaster. Evolution 46:76–91
Robert KA, Brunet-Rossinni A, Bronikowski AM (2007) Testing the ‘free radical theory of aging’ hypothesis: physiological differences in long-lived and short-lived colubrid snakes. Aging Cell 6:395–404
Rose MR (1984) Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution 38:1004–1010
Rose MR, Vu LN, Park SU, Graves JL Jr (1992) Selection on stress resistance increases longevity in Drosophila melanogaster. Exp Gerontol 27:241–250
Rothenfluh A, Heberlein U (2002) Drugs, flies, and videotape: the effects of ethanol and cocaine on Drosophila locomotion. Curr Opin Neurobiol 12:1–7
Sanz A, Fernandez-Ayala DJM, Stefanatos RKA, Jacobs HT (2010) Mitochondrial ROS production correlates with, but does not directly regulate lifespan in Drosophila. Aging 2:200–223
Sarup P, Sorensen P, Loeschcke V (2011) Flies selected for longevity retain a young gene expression profile. Age 33:69–80
Service PM (1987) Physiological mechanisms of increased stress resistance in Drosophila melanogaster selected for postponed senescence. Physiol Zool 60:321–326
Service PM, Hutchinson EW, Mackinley MD, Rose MR (1985) Resistance to environmental stress in Drosophila melanogaster selected for postponed senescence. Physiol Zool 58:380–389
Simon AF, Liang DT, Krantz DE (2006) Differential decline in behavioral performance of Drosophila melanogaster with age. Mech Ageing Dev 127:647–651
Sohal RS, Mockett RJ, Orr WC (2002) Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med 33:575–586
Sun AY, Ingelman-Sundberg M, Neve E, Matsumoto H, Nishitani Y, Minowa Y, Fukui Y, Bailey SM, Patel VB, Cunningham CC, Zima T, Fialova L, Mikulikova L, Popov P, Malbohan I, Janebova M, Nespor K, Sun GY (2001) Ethanol and oxidative stress. Alcohol Clin Exp Res 25:237S–243S
Thomson MS, Jacobson JW, Laurie CC (1991) Comparison of alcohol dehydrogenase expression in Drosophila melanogaster and D. simulans. Mol Biol Evol 8:31–48
Vallee BL, Hoch FL (1955) Zinc, a component of yeast alcohol dehydrogenase. Proc Natl Acad Sci 41:327–338
Vermeulen CJ, Loeschcke V (2007) Longevity and the stress response in Drosophila. Exp Gerontol 42:153–159
Williams GC (1957) Pleiotropy, natural selection, and the evolution of senescence. Evolution 11:398–411
Wit J, Sarup P, Lupsa N, Malte H, Frydenberg J, Loeschcke V (2013) Longevity for free? increased reproduction with limited trade-offs in Drosophila melanogaster selected for increased lifespan. Exp Gerontol 48:349–357
Zhang L, Ran Y, Wu M, Wang L (2009) Changes of the lifespan and certain biochemical indexes on Drosophila melanogaster by ethanol exposure. Wei Sheng Yan Jiu 38:144–147
Acknowledgements
The first and second authors thank the Department of Science and Technology, Government of India, for the financial support under the INSPIRE Program. The authors thank the Chairpersons of the Department of Studies in Zoology for the facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deepashree, S., Niveditha, S., Shivanandappa, T. et al. Oxidative stress resistance as a factor in aging: evidence from an extended longevity phenotype of Drosophila melanogaster. Biogerontology 20, 497–513 (2019). https://doi.org/10.1007/s10522-019-09812-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-019-09812-7