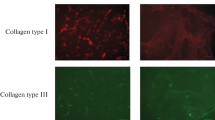
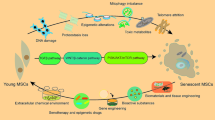
Cell senescence leads to changes in the secretory activity of mesenchymal stem cells (MSC), including proteins of extracellular matrix (ECM). Here we studied the regulatory properties of ECM of senescent MSC in a model with endothelial cells (EC). EC were seeded onto a decellularized extracellular matrix of senescent MSC. Changes in cell morphology and a decrease in cell growth were observed. In addition, increased production of inflammatory chemokines MCP-1 and GROα and reduced synthesis of proangiogenic growth factor FGF-2 were revealed. Analysis of ECM showed quantitative and qualitative changes, including fibronectin layer morphology, total protein content, and concentration of deposited growth factors such as VEGF. Thus, our work demonstrates that senescence of MSC can lead to modification of the effects of their ECM on EC activity.
Similar content being viewed by others
References
Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen. Med. 2019;4:22. https://doi.org/10.1038/s41536-019-0083-6
Kehl D, Generali M, Mallone A, Heller M, Uldry AC, Cheng P, Gantenbein B, Hoerstrup SP, Weber B. Proteomic analysis of human mesenchymal stromal cell secretomes: a systematic comparison of the angiogenic potential. NPJ Regen. Med. 2019;4:8. https://doi.org/10.1038/s41536-019-0070-y
Turinetto V, Vitale E, Giachino C. Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 2016;17(7):1164. https://doi.org/10.3390/ijms17071164
Lunyak VV, Amaro-Ortiz A, Gaur M. Mesenchymal stem cells secretory responses: senescence messaging secretome and immunomodulation perspective. Front. Genet. 2017;8:220. https://doi.org/10.3389/fgene.2017.00220
Ratushnyy A, Ezdakova M, Buravkova L. Secretome of senescent adipose-derived mesenchymal stem cells negatively regulates angiogenesis. Int. J. Mol. Sci. 2020;21(5):1802. https://doi.org/10.3390/ijms21051802
Ratushnyy AY, Buravkova LB. Cell senescence and mesenchymal stromal cells. Hum. Physiol. 2020;46(1):85-93. https://doi.org/10.1134/S0362119720010132
Ghosh D, Mejia Pena C, Quach N, Xuan B, Lee AH, Dawson MR. Senescent mesenchymal stem cells remodel extracellular matrix driving breast cancer cells to a more-invasive phenotype. J. Cell Sci. 2020;133(2):jcs232470. https://doi.org/10.1242/jcs.232470
Bertolo A, Baur M, Guerrero J, Pötzel T, Stoyanov J. Autofluorescence is a reliable in vitro marker of cellular senescence in human mesenchymal stromal cells. Sci. Rep. 2019;9(1):2074. https://doi.org/10.1038/s41598-019-38546-2
Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28(6):436-453. https://doi.org/10.1016/j.tcb.2018.02.001
Frescas D, Roux CM, Aygun-Sunar S, Gleiberman AS, Krasnov P, Kurnasov OV, Strom E, Virtuoso LP, Wrobel M, Osterman AL, Antoch MP, Mett V, Chernova OB, Gudkov AV. Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc. Natl Acad. Sci. USA. 2017;114(9):E1668-E1677. https://doi.org/10.1073/pnas.1614661114
Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513-522. https://doi.org/10.1016/j.cell.2005.02.003
Mavrogonatou E, Pratsinis H, Papadopoulou A, Karamanos NK, Kletsas D. Extracellular matrix alterations in senescent cells and their significance in tissue homeostasis. Matrix Biol. 2019;75-76:27-42. https://doi.org/10.1016/j.matbio.2017.10.004
Selman M, Pardo A. From pulmonary fibrosis to progressive pulmonary fibrosis: a lethal pathobiological jump. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021;321(3):L600-L607. https://doi.org/10.1152/ajplung.00310.2021
Bauer AL, Jackson TL, Jiang Y. Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS Comput. Biol. 2009;5(7):e1000445. https://doi.org/10.1371/journal.pcbi.1000445
Shibuya M. Vascular Endothelial Growth Factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2(12):1097-1105. https://doi.org/10.1177/1947601911423031
Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013;273(2):114-127. https://doi.org/10.1111/joim.12019
Liu L, Ratner BD, Sage EH, Jiang S. Endothelial cell migration on surface-density gradients of fibronectin, VEGF, or both proteins. Langmuir. 2007;23(22):11 168-11 173. https://doi.org/10.1021/la701435x
Claffey KP, Abrams K, Shih SC, Brown LF, Mullen A, Keough M. Fibroblast growth factor 2 activation of stromal cell vascular endothelial growth factor expression and angiogenesis. Lab. Invest. 2001;81(1):61-75. https://doi.org/10.1038/labinvest.3780212
Ponticos M. Connective tissue growth factor (CCN2) in blood vessels. Vascul. Pharmacol. 2013;58(3):189-193. https://doi.org/10.1016/j.vph.2013.01.004
Ungvari Z, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Fülöp GA, Kiss T, Csiszar A. Connective tissue growth factor (CTGF) in age-related vascular pathologies. Geroscience. 2017;39(5-6):491-498. https://doi.org/10.1007/s11357-017-9995-5
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 2, pp. 119-125, June, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matveeva, D.K., Ezdakova, M.I. & Ratushnyy, A.Y. Modification of the Properties of Extracellular Matrix of Senescent Mesenchymal Stem Cells. Bull Exp Biol Med 175, 569–575 (2023). https://doi.org/10.1007/s10517-023-05905-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-023-05905-z