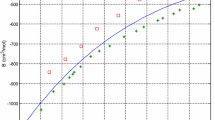
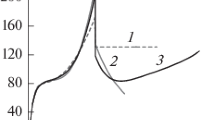
It is shown that the thermodynamics characteristics of uranium hexafluoride solutions with volatile hexaand pentafluoride elements are a consequence of the strictly symmetric configuration of their molecular structure in the gaseous and liquid states. Simple methods are proposed for evaluating the properties of binary mixtures of elemental penta- and hexafluorides. These methods are based on a comparison of the element–fluorine bond length in the constituent molecules of system and the known enthalpy of mixing of one solution with the potential energy in the formation of a pair of unlike molecules of a different solution.
Similar content being viewed by others
References
A. Ponelis, M. Slabber, and C. Zimmer, Conversion of Non-Nuclear Grade Feedstock to UF 4 : Advances in Uranium Refining and Conversion, IAEA-TECDOC-420, IAEA, Vienna (1987), pp. 105–140.
V. K. Ezhov, “Commercial rectification facility for deep purification of uranium hexafluoride sublimate,” At. Énerg., 103, No. 5, 314–318 (2007).
A. N. Shubin, V. D. Michurov, V. K. Mustafaev, and V. K. Ezhov, “Separation of enriched uranium hexafluoride in a pilot rectification facility,” At. Énerg., 103, No. 3, 179–181 (2007).
K. A. Ledovskikh and V. A. Khokhlov, “Rectification facility for purifying high-enrichment uranium hexafluoride,” Abstr. 9th Int. Student Conf. Northern Lights 2006, St. Petersburg (2006), pp. 26–27.
V. V. Kafarov, Methods of Cybernetics in Chemistry and Chemical Technology, Khimiya, Moscow (1985).
Zh. A. Bril, “Mathematical modeling of liquid–vapor phase equilibria of multicomponent mixtures I,” Zh. Fiz. Khim., 47, No. 10, 2009–2015 (1973).
V. T. Zharov and L. A. Serafi mov, Physicochemical Principles of Distillation and Rectifi cation, Khimiya, Moscow (1975).
I. A. Uspenskaya, Summary of Lectures on Physical Chemistry, Izd. MGU Lomonosova, Moscow (2005).
E. Hala, J. Pick, V. Fried, and O. Vilim, Vapor-Liquid Equilibrium [Russian translation], Izd. Inostr. Lit., Moscow (1962).
M. Kh. Karapet’yants, Methods of Comparative Calculations of Physicochemical Properties, Nauka, Moscow (1965).
V. N. Prusakov and V. K. Ezhov, “Investigations on the reprocessing of spent fuel,” in: Phase Diagram of Binary Systems of Uranium Hexafluoride with Higher Fluorides of Tungsten, Molybdenum, and Niobium, Atomic Energy Commission, Czechoslovakia (1968), pp. 331–348.
V. K. Ezhov, “Solubility of uranium hexafluoride in high fluorides of group-V and -VI metals,” Abstr. 5th All-Union Symp. Chemistry of Inorganic Fluorides, Dnepropetrovsk (1978), p. 106.
V. K. Ezhov, “Polymerization of molecules and stratification in binary systems of metal penta- and hexafl uorides,” ibid., p.105.
B. Weinsnock, “The 25-year revolution in hexafluoride Chemistry,” Chem. Eng., 9, 86–100 (1964).
B. Weinstock and G. L. Goodman, “Vibrational properties of hexafluoride molecules,” Adv. Chem. Phys., 9, 169–176 (1965).
O. P. Chalkin, “Stability and structure of gaseous molecules of metal fluorides,” Zh. Neorg. Khim., 18, No. 9, 2307–2317(1973).
G. V. Romanov and V. P. Spiridonov, “Electron diffraction investigation of vanadium pentafluoride in vapors,” Zh. Strukt. Khim., 7, No. 6, 882–883 (1966).
R. Peacock, “Transition metals pentafluorides and related compounds,” Adv. Fluor. Chem., 7, 113 –145 (1973).
V. K. Ezhov, “Investigation of the system uranium hexafluoride–antimony pentafluoride,” Zh. Neorg. Khim., 17, No. 7, 2043–2045 (1972).
J. O. Hirschfelder, C. F. Curtiss, and R. B. Bird, Molecular Theory of Gases and Liquids [Russian translation], Izd. Inostr. Lit., Moscow (1961).
N. A. Smirnova, Molecular Theory of Solutions, Khimiya, Leningrad (1987).
M. I. Shakhparonov, Introduction to the Molecular Theory of Solutions, Gos. Izd. Nauch.-Teor. Lit., Moscow (1956).
L. V. Gurvich, Rupture Energy of the Chemical Bond, Nauka, Moscow (1974).
I. Bretschneider, Physical Properties of Liquids and Gases, Nauka, Moscow (1969).
S. S. Batsanov, Electronegativity of the Elements and Chemical Bonds, Izd. SO AN SSSR, Novosibirsk (1962).
M. Kh. Karapet’yants, Chemical Thermodynamics, Khimiya, Moscow (1975).
I. G. Ryss, The Chemistry of Fluorine and Its Inorganic Compounds, Goskhimizdat, Moscow (1956).
E. A. Moelwyn-Hughes, Physical Chemistry [Russian translation], Izd. Inostr. Lit., Moscow (1962), Vol. 2.
G. Seaborg and J. Katz, Chemistry of Actinide Elements [Russian translation], Atomizdat, Moscow (1960).
Author information
Authors and Affiliations
Additional information
Translated from Atomnaya Énergiya, Vol. 120, No. 1, pp. 37–42, January, 2016.
Rights and permissions
About this article
Cite this article
Ezhov, V.K. Comparative Method of Calculating the Thermodynamic Parameters of Some Uranium Hexafluoride Solutions. At Energy 120, 48–54 (2016). https://doi.org/10.1007/s10512-016-0094-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10512-016-0094-y