Abstract
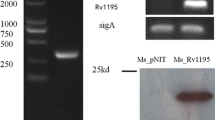
Mycobacterium tuberculosis (Mtb) genome possesses a unique family called Proline-Glutamate/Proline-Proline-Glutamate (PE/PPE) gene family, exclusive to pathogenic mycobacterium. Some of these proteins are known to play role in virulence and immune response modulation, but many are still uncharacterized. This study investigated the role of C-terminal region of Rv1039c (PPE15) in inducing mitochondrial perturbations and macrophage apoptosis. Our in-silico studies revealed the disordered, coiled, and hydrophobic C-terminal region in Rv1039c has similarity with C-terminal of mitochondria-targeting pro-apoptotic host proteins. Wild type Rv1039c and C-terminal deleted Rv1039c (Rv1039c-/-Cterm) recombinant proteins were purified and their M. smegmatis knock-in strains were constructed which were used for in-vitro experiments. Confocal microscopy showed localization of Rv1039c to mitochondria of PMA-differentiated THP1 macrophages; and reduced mitochondrial membrane depolarization and production of mitochondrial superoxides were observed in response to Rv1039c-/-Cterm in comparison to full-length Rv1039c. The C-terminal region of Rv1039c was found to activate caspases 3, 7 and 9 along with upregulated expression of pro-apoptotic genes like Bax and Bim. Rv1039c-/-Cterm also reduced the Cytochrome-C release from the mitochondria and the expression of AnnexinV/PI positive and TUNEL positive cells as compared to Rv1039c. Additionally, Rv1039c was observed to upregulate the TLR4-NF-κB-TNF-α signalling whereas the same was downregulated in response to Rv1039c-/-Cterm. These findings suggested that the C-terminal region of Rv1039c is a molecular mimic of pro-apoptotic host proteins which induce mitochondria-dependent macrophage apoptosis and evoke host immune response. These observations enhance our understanding about the role of PE/PPE proteins at host-pathogen interface.













Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary data files). Further enquiries related to the study can be directed to the corresponding author.
References
Liu CH, Liu H, Ge B (2017) Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol 14:963–975. https://doi.org/10.1038/cmi.2017.88
Ahmad J, Khubaib M, Sheikh JA, Pancsa R, Kumar S, Srinivasan A, Babu MM, Hasnain SE, Ehtesham NZ (2020) Disorder-to-order transition in PE-PPE proteins of Mycobacterium tuberculosis augments the pro-pathogen immune response. FEBS Open Bio 10:70–85. https://doi.org/10.1002/2211-5463.12749
Ates LS (2020) New insights into the mycobacterial PE and PPE proteins provide a framework for future research. Mol Microbiol 113. https://doi.org/10.1111/mmi.14409
Chai Q, Wang X, Qiang L, Zhang Y, Ge P, Lu Z, Zhong Y, Li B, Wang J, Zhang L, Zhou D, Li W, Dong W, Pang Y, Gao GF, Liu CH (2019) A Mycobacterium tuberculosis surface protein recruits ubiquitin to trigger host xenophagy. Nat Commun 10:1973. https://doi.org/10.1038/s41467-019-09955-8
Ahmad J, Farhana A, Pancsa R, Arora SK, Srinivasan A, Tyagi AK, Babu MM, Ehtesham NZ, Hasnain SE (2018) Contrasting Function of Structured N-Terminal and Unstructured C-Terminal Segments of Mycobacterium tuberculosis PPE37 Protein., MBio. 9 https://doi.org/10.1128/mBio.01712-17
Srivastava S, Battu MB, Khan MZ, Nandicoori VK, Mukhopadhyay S (2019) Mycobacterium tuberculosis PPE2 protein interacts with p67(phox) and inhibits reactive oxygen species production. J Immunol 203:1218–1229. https://doi.org/10.4049/jimmunol.1801143
Medha, Priyanka P, Bhatt S, Sharma M, Sharma (2022) Role of C-terminal domain of Mycobacterium tuberculosis PE6 (Rv0335c) protein in host mitochondrial stress and macrophage apoptosis, apoptosis. https://doi.org/10.1007/s10495-022-01778-1
Sharma S, Schiller MR (2019) The carboxy-terminus, a key regulator of protein function. Crit Rev Biochem Mol Biol 54:85–102. https://doi.org/10.1080/10409238.2019.1586828
Gómez-Fernández JC (2014) Functions of the C-terminal domains of apoptosis-related proteins of the Bcl-2 family. Chem Phys Lipids 183:77–90. https://doi.org/10.1016/j.chemphyslip.2014.05.003
Peng Z, Xue B, Kurgan L, Uversky VN (2013) Resilience of death: intrinsic disorder in proteins involved in the programmed cell death. Cell Death Differ 20:1257–1267. https://doi.org/10.1038/cdd.2013.65
Wang J, Ge P, Lei Z, Lu Z, Qiang L, Chai Q, Zhang Y, Zhao D, Li B, Su J, Peng R, Pang Y, Shi Y, Zhang Y, Gao GF, Qiu X, Liu CH (2021) Mycobacterium tuberculosis protein kinase G acts as an unusual ubiquitinating enzyme to impair host immunity. EMBO Rep 22:e52175. https://doi.org/10.15252/embr.202052175
Lucattini R, Likić VA, Lithgow T (2004) Bacterial proteins predisposed for targeting to Mitochondria. Mol Biol Evol 21:652–658. https://doi.org/10.1093/molbev/msh058
Grover S, Sharma T, Singh Y, Kohli S, Singh MPA, Semmler T, Wieler LH, Tedin K, Ehtesham NZ, Hasnain SE (2018) The PGRS Domain of Mycobacterium tuberculosis PE_PGRS Protein Rv0297 Is Involved in Endoplasmic Reticulum Stress-Mediated Apoptosis through Toll-Like Receptor 4., MBio. 9 https://doi.org/10.1128/mBio.01017-18
Cadieux N, Parra M, Cohen H, Maric D, Morris SL, Brennan MJ (2011) Induction of cell death after localization to the host cell mitochondria by the Mycobacterium tuberculosis PE-PGRS33 protein. Microbiology 157:793–804. https://doi.org/10.1099/mic.0.041996-0
Sharma N, Shariq M, Quadir N, Singh J, Sheikh JA, Hasnain SE, Ehtesham NZ (2021) Mycobacterium tuberculosis protein PE6 (Rv0335c), a Novel TLR4 agonist, evokes an inflammatory response and modulates the cell death pathways in macrophages to enhance intracellular survival. Front Immunol 12:696491. https://doi.org/10.3389/fimmu.2021.696491
Priyanka, Medha P, Bhatt H, Joshi S, Sharma M, Sharma (2023) Late stage specific Rv0109 (PE_PGRS1) protein of Mycobacterium tuberculosis induces mitochondria mediated macrophage apoptosis. Microb Pathog 176:106021. https://doi.org/10.1016/j.micpath.2023.106021
Martin M, DeVisch A, Boudehen Y-M, Barthe P, Turapov O, Aydogan T, Heriaud L, Gracy J, Mukamolova GV, Letourneur F, Cohen-Gonsaud M (2021) A Mycobacterium tuberculosis effector targets mitochondrion, controls energy metabolism and limits cytochrome c exit, BioRxiv. 2021.01.31.428746. https://doi.org/10.1101/2021.01.31.428746
Duan L, Gan H, Golan DE, Remold HG (2002) Critical role of mitochondrial damage in determining outcome of macrophage infection with Mycobacterium tuberculosis. J Immunol 169:5181–5187. https://doi.org/10.4049/jimmunol.169.9.5181
Jamwal S, Midha MK, Verma HN, Basu A, Rao KVS, Manivel V (2013) Characterizing virulence-specific perturbations in the mitochondrial function of macrophages infected with Mycobacterium tuberculosis. Sci Rep 3. https://doi.org/10.1038/srep01328
Lorenzo HK, Susin SA (2004) Mitochondrial effectors in caspase-independent cell death. FEBS Lett 557:14–20. https://doi.org/10.1016/s0014-5793(03)01464-9
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94:909–950. https://doi.org/10.1152/physrev.00026.2013
Zhu Y, Wan C, Lin J, Hammes H (2022) Mitochondrial Oxidative Stress and Cell Death in Podocytopathies
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629. https://doi.org/10.1126/science.1099320
Medha, Priyanka S, Sharma M, Sharma (2023) PE_PGRS45 (Rv2615c) protein of Mycobacterium tuberculosis perturbs mitochondria of macrophages. Immunol Cell Biol. https://doi.org/10.1111/imcb.12677
Mossmann D, Meisinger C, Vögtle F-N (2012) Processing of mitochondrial presequences. Biochim Biophys Acta 1819:1098–1106. https://doi.org/10.1016/j.bbagrm.2011.11.007
Davis JM, Ramakrishnan L (2009) The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136:37–49. https://doi.org/10.1016/j.cell.2008.11.014
Daniel J, Kapoor N, Sirakova T, Sinha R, Kolattukudy P (2016) The perilipin-like PPE15 protein in Mycobacterium tuberculosis is required for triacylglycerol accumulation under dormancy-inducing conditions. Mol Microbiol 101:784–794. https://doi.org/10.1111/mmi.13422
Paysan-Lafosse T, Blum M, Chuguransky S, Grego T, Pinto BL, Salazar GA, Bileschi ML, Bork P, Bridge A, Colwell L, Gough J, Haft DH, Letunić I, Marchler-Bauer A, Mi H, Natale DA, Orengo CA, Pandurangan AP, Rivoire C, Sigrist CJA, Sillitoe I, Thanki N, Thomas PD, Tosatto SCE, Wu CH, Bateman A (2023) Nucleic Acids Res 51:D418–D427 InterPro in 2022. https://doi.org/10.1093/nar/gkac993
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. https://doi.org/10.1093/nar/22.22.4673
Qian J, Chen R, Wang H, Zhang X (2020) Role of the PE/PPE family in Host-Pathogen interactions and prospects for Anti-tuberculosis Vaccine and Diagnostic Tool Design. Front Cell Infect Microbiol 10:594288. https://doi.org/10.3389/fcimb.2020.594288
Danelishvili L, Everman J, Bermudez LE Mycobacterium tuberculosis PPE68 and Rv2626c genes contribute to the host cell necrosis and bacterial escape from macrophages. Virulence 7 (2016) 23–32. https://doi.org/10.1080/21505594.2015.1102832
Joseph S, Yuen A, Singh V, Hmama Z (2017) Mycobacterium tuberculosis Cpn60.2 (GroEL2) blocks macrophage apoptosis via interaction with mitochondrial mortalin. Biol Open 6:481–488. https://doi.org/10.1242/bio.023119
Dubey RK, Dhamija E, Kumar Mishra A, Soam D, Mohanrao Yabaji S, Srivastava K, Srivastava KK (2021) Mycobacterial origin protein Rv0674 localizes into mitochondria, interacts with D-loop and regulates OXPHOS for intracellular persistence of Mycobacterium tuberculosis. Mitochondrion 57:241–256. https://doi.org/10.1016/j.mito.2020.11.014
Wang C, Youle RJ (2009) The role of mitochondria in apoptosis*. Annu Rev Genet 43:95–118. https://doi.org/10.1146/annurev-genet-102108-134850
Kruh NA, Troudt J, Izzo A, Prenni J, Dobos KM (2010) Portrait of a Pathogen: the Mycobacterium tuberculosis Proteome in vivo. PLoS ONE 5:e13938
Murphy DJ, Brown JR (2007) Identification of gene targets against dormant phase Mycobacterium tuberculosis infections. BMC Infect Dis 7:84. https://doi.org/10.1186/1471-2334-7-84
Gillies LA, Kuwana T (2014) Apoptosis regulation at the mitochondrial outer membrane. J Cell Biochem 115:632–640. https://doi.org/10.1002/jcb.24709
Mohareer K, Medikonda J, Vadankula GR (2020) Mycobacterial Control of Host Mitochondria: Bioenergetic and metabolic changes shaping cell fate and infection outcome. 10. https://doi.org/10.3389/fcimb.2020.00457
Sohn H, Kim JS, Shin SJ, Kim K, Won CJ, Kim WS, Min KN, Choi HG, Lee JC, Park JK, Kim HJ (2011) Targeting of Mycobacterium tuberculosis heparin-binding hemagglutinin to mitochondria in macrophages. PLoS Pathog 7:1–17. https://doi.org/10.1371/journal.ppat.1002435
Sánchez A, Espinosa P, García T, Mancilla R (2012) The 19 kDa mycobacterium tuberculosis lipoprotein (lpqh) induces macrophage apoptosis through extrinsic and intrinsic pathways: A role for the mitochondrial apoptosis-inducing factor. Clin Dev Immunol 2012. https://doi.org/10.1155/2012/950503
Lee K, Choi S, Choi H, Gurmessa S (2020) Recombinant Rv3261 protein of Mycobacterium tuberculosis induces apoptosis through a mitochondrion-dependent pathway in macrophages and inhibits intracellular bacterial growth. Cell Immunol 354:104145. https://doi.org/10.1016/j.cellimm.2020.104145
Aguiló N, Uranga S, Marinova D, Martín C, Pardo J (2014) Bim is a crucial regulator of apoptosis induced by Mycobacterium tuberculosis. Cell Death Dis 5:e1343. https://doi.org/10.1038/cddis.2014.313
Ahmed A, Dolasia K, Mukhopadhyay S (2018) Mycobacterium tuberculosis PPE18 protein reduces inflammation and increases Survival in Animal Model of Sepsis. J Immunol 200:3587–3598. https://doi.org/10.4049/jimmunol.1602065
Deng W, Yang W, Zeng J, Abdalla AE, Xie J (2016) Mycobacterium tuberculosis PPE32 promotes cytokines production and host cell apoptosis through caspase cascade accompanying with enhanced ER stress response. Oncotarget 7:67347–67359. https://doi.org/10.18632/oncotarget.12030
Daim S, Kawamura I, Tsuchiya K, Hara H, Kurenuma T, Shen Y, Dewamitta SR, Sakai S, Nomura T, Qu H, Mitsuyama M (2011) Expression of the Mycobacterium tuberculosis PPE37 protein in Mycobacterium smegmatis induces low tumour necrosis factor alpha and interleukin 6 production in murine macrophages. J Med Microbiol 60:582–591. https://doi.org/10.1099/jmm.0.026047-0
Akira S (2009) Pathogen recognition by innate immunity and its signaling., Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 85 143–156. https://doi.org/10.2183/pjab.85.143
Harding CV, Boom WH (2010) Regulation of antigen presentation by Mycobacterium tuberculosis: a role for toll-like receptors. Nat Rev Microbiol 8:296–307. https://doi.org/10.1038/nrmicro2321
Brennan MJ (2017) The enigmatic PE/PPE Multigene Family of Mycobacteria and Tuberculosis Vaccination. Infect Immun 85:e00969–e00916. https://doi.org/10.1128/IAI.00969-16
Sharma T, Grover S, Arora N, P M, Ehtesham NZ, Hasnain SE (2020) PGRS Domain of Rv0297 of Mycobacterium tuberculosis is involved in modulation of macrophage functions to Favor Bacterial persistence. Front Cell Infect Microbiol 10:451. https://doi.org/10.3389/fcimb.2020.00451
Tiwari B, Ramakrishnan UM, Raghunand TR (2015) The Mycobacterium tuberculosis protein pair PE9 (Rv1088)-PE10 (Rv1089) forms heterodimers and induces macrophage apoptosis through toll-like receptor 4. Cell Microbiol 17:1653–1669. https://doi.org/10.1111/cmi.12462
Acknowledgements
We gratefully acknowledge Professor Vikas Jain of Indian Institute of Science Education and Research [IISER], Bhopal for providing the Rv1039c-/-Cterm clone in pMSQSCHS vector.
Funding
The financial support by Department of Science and Technology [DST], Government of India [Grant Sanction no. EMR/2016/006774] and Intramural Research grant from MH, R&D cell is gratefully acknowledged. Priyanka is recipient of Senior Research Fellowship from Council of Scientific and Industrial Research [CSIR], Government of India.
Author information
Authors and Affiliations
Contributions
P: Experimental design, Methodology, Investigation, Formal analysis, Data curation, Writing-original draft, Writing-review and editing. SS: Supervision, Funding acquisition, Review and editing. MVB: Supervision in M. smegmatis experiments. MS: Conceptualization, Experiment design, Methodology, Supervision, Writing-review and editing of manuscript, Funding acquisition, Investigation.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Priyanka, Sharma, S., Varma-Basil, M. et al. C-terminal region of Rv1039c (PPE15) protein of Mycobacterium tuberculosis targets host mitochondria to induce macrophage apoptosis. Apoptosis (2024). https://doi.org/10.1007/s10495-024-01965-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s10495-024-01965-2