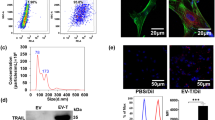
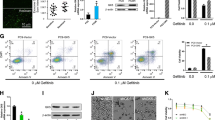
Abstract
The aberrantly up-regulated CDK9 can be targeted for cancer therapy. The CDK inhibitor dinaciclib (Dina) has been found to drastically sensitizes cancer response to TRAIL-expressing extracellular vesicle (EV-T). However, the low selectivity of Dina has limited its application for cancer. We propose that CDK9-targeted siRNA (siCDK9) may be a good alternative to Dina. The siCDK9 molecules were encapsulated into EV-Ts to prepare a complexed nanodrug (siEV-T). It was shown to efficiently suppress CDK9 expression and overcome TRAIL resistance to induce strikingly augmented apoptosis in lung cancer both in vitro and in vivo, with a mechanism related to suppression of both anti-apoptotic factors and nuclear factor-kappa B pathway. Therefore, siEV-T potentially constitutes a novel, highly effective and safe therapy for cancers.







Similar content being viewed by others
Data availability
Not applicable.
References
Kumar SK et al (2015) Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 125(3):443–448
Hortobagyi GN et al (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375(18):1738–1748
Serra F et al (2019) Palbociclib in metastatic breast cancer: current evidence and real-life data. Drugs Context 8:212579
Wang Y, Liu XY, De Clercq E (2009) Role of the HIV-1 positive elongation factor P-TEFb and inhibitors thereof. Mini Rev Med Chem 9(3):379–385
Mandal R, Becker S, Strebhardt K (2021) Targeting CDK9 for anti-cancer therapeutics. Cancers (Basel) 13:9
Abdalla Y et al (2021) Safranal prevents liver cancer through inhibiting oxidative stress and alleviating inflammation. Front Pharmacol 12:777500
Amin A et al (2011) Pancreas-protective effects of chlorella in STZ-induced diabetic animal model: insights into the mechanism. J Diabet Mell 1(3):36–45
El-Dakhly SM et al (2020) Aescin and diosmin each alone or in low dose- combination ameliorate liver damage induced by carbon tetrachloride in rats. BMC Res Notes 13(1):259
Abdel-Latif R et al (2022) TLRs-JNK/ NF-κB pathway underlies the protective effect of the sulfide salt against liver toxicity. Front Pharmacol 13:850066
Ke C et al (2020) Extracellular vesicle delivery of TRAIL eradicates resistant tumor growth in combination with CDK inhibition by dinaciclib. Cancers (Basel) 12:5
Lemke J et al (2014) Selective CDK9 inhibition overcomes TRAIL resistance by concomitant suppression of cFlip and Mcl-1. Cell Death Differ 21(3):491–502
Wang HK (2001) Flavopiridol. National cancer institute. Curr Opin Investig Drugs 2(8):1149–1155
Blachly JS, Byrd JC (2013) Emerging drug profile: cyclin-dependent kinase inhibitors. Leuk Lymphoma 54(10):2133–2143
Anshabo AT et al (2021) CDK9: a comprehensive review of its biology, and its role as a potential target for anti-cancer agents. Front Oncol 11:678559
Gojo I et al (2013) Clinical and laboratory studies of the novel cyclin-dependent kinase inhibitor dinaciclib (SCH 727965) in acute leukemias. Cancer Chemother Pharmacol 72(4):897–908
Fabre C et al (2014) Clinical study of the novel cyclin-dependent kinase inhibitor dinaciclib in combination with rituximab in relapsed/refractory chronic lymphocytic leukemia patients. Cancer Chemother Pharmacol 74(5):1057–1064
Parry D et al (2010) Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther 9(8):2344–2353
Crean S et al (2009) Safety of multi-targeted kinase inhibitors as monotherapy treatment of cancer: a systematic review of the literature. Curr Drug Saf 4(2):143–154
Goel B et al (2020) Small molecule CDK inhibitors for the therapeutic management of cancer. Curr Top Med Chem 20(17):1535–1563
Saw PE, Song EW (2020) siRNA therapeutics: a clinical reality. Sci China Life Sci 63(4):485–500
Kaur K et al (2018) Chemotherapy with si-RNA and anti-cancer drugs. Curr Drug Deliv 15(3):300–311
Mendt M et al (2018) Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 3:8
Hou H et al (2021) TRAIL-Armed ER nanosomes induce drastically enhanced apoptosis in resistant tumor in combination with the antagonist of IAPs (AZD5582). Adv Healthc Mater 10(11):e2100030
Ke C et al (2022) Extracellular vesicle-mediated co-delivery of TRAIL and dinaciclib for targeted therapy of resistant tumors. Biomater Sci 10(6):1498–1514
Higginbotham JN et al (2016) Identification and characterization of EGF receptor in individual exosomes by fluorescence-activated vesicle sorting. J Extracell Vesicles 5:29254
Ke C et al (2020) Extracellular vesicle delivery of TRAIL eradicates resistant tumor growth in combination with CDK inhibition by dinaciclib. Cancers 12:5
Levy JMM, Towers CG, Thorburn A (2017) Targeting autophagy in cancer. Nat Rev Cancer 17(9):528–542
Deng D, Shah K (2020) TRAIL of hope meeting resistance in cancer. Trends Cancer 6(12):989–1001
Yuan Z et al (2015) Mesenchymal stromal cell delivery of full-length tumor necrosis factor-related apoptosis-inducing ligand is superior to soluble type for cancer therapy. Cytotherapy 17(7):885–896
Huang C et al (2022) Sensitizing TRAIL response via differential modulation of anti- and pro-apoptotic factors by AZD5582 combined with ER nanosomal TRAIL in neuroblastoma. Acta Histochem 124(2):151856
Paparidis NF, Durvale MC, Canduri F (2017) The emerging picture of CDK9/P-TEFb: more than 20 years of advances since PITALRE. Mol Biosyst 13(2):246–276
He S et al (2020) Targeting CDK9: a novel biomarker in the treatment of endometrial cancer. Oncol Rep 44(5):1929–1938
Sengupta S, Biarnes MC, Jordan VC (2014) Cyclin dependent kinase-9 mediated transcriptional de-regulation of cMYC as a critical determinant of endocrine-therapy resistance in breast cancers. Breast Cancer Res Treat 143(1):113–124
Maguire O, O’Loughlin K, Minderman H (2015) Simultaneous assessment of NF-κB/p65 phosphorylation and nuclear localization using imaging flow cytometry. J Immunol Methods 423:3–11
Ghosh S, May MJ, Kopp EB (1998) NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16:225–260
Tilborghs S et al (2017) The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit Rev Oncol Hematol 120:141–150
Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5(10):749–759
Peng C et al (2020) The NF-κB signaling pathway, the microbiota, and gastrointestinal tumorigenesis: recent advances. Front Immunol 11:1387
Rasmi RR, Sakthivel KM, Guruvayoorappan C (2020) NF-κB inhibitors in treatment and prevention of lung cancer. Biomed Pharmacother 130:110569
Baldwin AS (2012) Regulation of cell death and autophagy by IKK and NF-κB: critical mechanisms in immune function and cancer. Immunol Rev 246(1):327–345
Katsman A, Umezawa K, Bonavida B (2009) Chemosensitization and immunosensitization of resistant cancer cells to apoptosis and inhibition of metastasis by the specific NF-kappaB inhibitor DHMEQ. Curr Pharm Des 15(7):792–808
Mi J et al (2007) NF-kappaB inhibition by an adenovirus expressed aptamer sensitizes TNFalpha-induced apoptosis. Biochem Biophys Res Commun 359(3):475–480
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43(6):582–592
Fan H et al (2022) Carbon nanoparticles induce DNA repair and PARP inhibitor resistance associated with nanozyme activity in cancer cells. Cancer Nanotechnology 13:1
Al Hrout A et al (2022) Modelling liver cancer microenvironment using a novel 3D culture system. Sci Rep 12(1):8003
Nelson DR et al (2022) Molecular mechanisms behind safranal’s toxicity to HepG2 cells from dual omics. Antioxidants (Basel) 11:6
Funding
Z.Q.Y. is a China Talented Scholar Scheme Research Fellow in the School of Biomedical and Pharmaceutical Sciences and is supported by National Natural Science Foundation of China (grant number 82173850), the University Innovative Team Support for Major Chronic Diseases and Drug Development (26330320901), and the Guangdong Provincial Talented Scholar Foundation (220418137).
Author information
Authors and Affiliations
Contributions
Q.Y., K.S., and S.Y.L. contributed equally to this work. Conceptualization, Q.Y., K.S., S.Y.L., and Z.Q.Y.; methodology, Q.Y.,K.S., X.Y.L., L.L.,C.H.K., W.J.S., Y.H., R.T., W.T.Z., Z.J.D., C.M.C., Q.J.L., J.N.Y., Z.H.W., formal analysis, Z.Q.Y., Q.Y., C.H.K., and M.H.Y.,; investigation, Q.Y., X.Y., W.Z.; resources, Z.Q.Y.,; writing-original draft preparation, Q.Y., and Z.Q.Y.; Funding Acquisition, Z.Q.Y.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Ethical approval
All procedures and protocols for in vivo study were approved by the Animal Ethics Committee in South China University of Technology (Approval ID: 20221020239; Date: February 2, 2022). All animal model experiments were carried out in accordance with the ethical standards outlined in the Best Practice Guidelines on Publishing Ethics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, Q., Su, K., Li, S. et al. Selective CDK9 knockdown sensitizes TRAIL response by suppression of antiapoptotic factors and NF-kappaB pathway. Apoptosis 28, 1060–1075 (2023). https://doi.org/10.1007/s10495-023-01842-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01842-4