Abstract
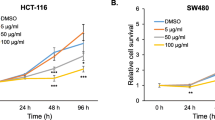
Colorectal carcinoma (CRC) is the third most prevalent cancer, causing a significant mortality worldwide. Present available therapies are surgery, chemotherapy including radiotherapy, and these are known to be associated with heavy side effects. Therefore, nutritional intervention in the form of natural polyphenols has been well recognised to prevent CRC. Phloretin, a known dihydrochalcone is present in apple, pear and strawberry. This has been proven to induce apoptosis in cancer cells and also exhibited anti-inflammatory activity, thus can be explored as a potential anticancer nutraceutical agent. This study demonstrated phloretin’s significant in vitro anticancer activity against CRC. Phloretin suppressed cell proliferation, colony forming ability and cellular migration in human colorectal cancer HCT-116 and SW-480 cells. Results also revealed that phloretin generated reactive oxygen species (ROS) which provoked depolarization of mitochondrial membrane potential (MMP) and further contributed to cytotoxicity in colon cancer cells. Phloretin also modulated the cell cycle regulators including cyclins and cyclin-dependent kinases (CDKs) and halted cell cycle at G2/M phase. Moreover, it also induced apoptosis by regulating the expression of Bax and BCl-2. The Wnt/β-catenin signaling is inactivated by phloretin by targeting the downstream oncogenes namely CyclinD1, c-Myc and Survivin which are involved in the proliferation and apoptosis of colon cancer cells. In our study we showed that lithium chloride (LiCl) induced the expression of β-catenin and its target genes and the co-treatment of phloretin circumvent its effect and downregulated the Wnt/β-catenin signaling. In conclusion, our results strongly suggested that phloretin can be utilized as a nutraceutical anticancer agent for combating CRC.
Graphical abstract













Similar content being viewed by others
Data availability
All the data generated or analyzed during the study are included in this article or are available upon request.
Abbreviations
- CDKs :
-
Cyclin dependent Kinases
- CRC :
-
Colorectal cancer
- DCFH:
-
-DA 2’-7’dichlorofluorescin diacetate
- DMEM :
-
Dulbecco’s modified eagle medium
- FBS :
-
Fetal Bovine serum
- IC50 :
-
Inhibitory concentration
- IEC-6:
-
Intestinal epithelial cells
- JC-1:
-
Tetraethylbenzimidazolylcarbocyanine iodide
- LiCl:
-
Lithium chloride
- MMP:
-
Mitochondrial membrane potential
- qRT-PCR:
-
Quantitative real time polymerase chain reaction
- ROS:
-
Reactive oxygen species
- SI:
-
Selectivity index
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Wild CP (2014) International agency for research on cancer. Encyclopedia of toxicology. Elsevier, Amsterdam, pp 1067–1069
Siegel RL, Miller KD, Goding Sauer A et al (2020) Colorectal cancer statistics, 2020. CA Cancer J Clin 70:145–164. https://doi.org/10.3322/caac.21601
Islami F, Goding Sauer A, Miller KD et al (2017) Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 68:31–54. https://doi.org/10.3322/caac.21440
Baena R, Salinas P (2015) Diet and colorectal cancer. Maturitas 80:258–264. https://doi.org/10.1016/j.maturitas.2014.12.017
Kunzmann AT, Coleman HG, Huang W-Y et al (2015) Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Clin Nutr 102:881–890. https://doi.org/10.3945/ajcn.115.113282
Thanikachalam K, Khan G (2019) Colorectal cancer and nutrition. Nutrients 11:164. https://doi.org/10.3390/nu11010164
Akiyama Y, Kimura Y, Enatsu R et al (2018) Advantages and disadvantages of combined chemotherapy with carmustine wafer and bevacizumab in patients with newly diagnosed glioblastoma: a single-institutional experience. World Neurosurg 113:e508–e514. https://doi.org/10.1016/j.wneu.2018.02.070
Bar-Shalom R, Bergman M, Grossman S et al (2019) Inula viscosa extract inhibits growth of colorectal cancer cells in vitro and in vivo through induction of apoptosis. Front Oncol 9:227. https://doi.org/10.3389/fonc.2019.00227
Fouad MA, Agha AM, Merzabani MA, Shouman SA (2013) Resveratrol inhibits proliferation, angiogenesis and induces apoptosis in colon cancer cells. Hum & Exp Toxicol 32:1067–1080. https://doi.org/10.1177/0960327113475679
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47–e47. https://doi.org/10.1017/jns.2016.41
Signorelli P, Fabiani C, Brizzolari A et al (2015) Natural grape extracts regulate colon cancer cells malignancy. Nutr Cancer 67:494–503. https://doi.org/10.1080/01635581.2015.1004591
Long J, Guan P, Hu X et al (2021) Natural polyphenols as targeted modulators in colon cancer: molecular mechanisms and applications. Front Immunol. https://doi.org/10.3389/fimmu.2021.635484
Rana S, Bhushan S (2016) Apple phenolics as nutraceuticals: assessment, analysis and application. J Food Sci Technol 53:1727–1738. https://doi.org/10.1007/s13197-015-2093-8
Jaganathan SK, Vellayappan MV, Narasimhan G et al (2014) Chemopreventive effect of apple and berry fruits against colon cancer. World J Gastroenterol 20:17029–17036. https://doi.org/10.3748/wjg.v20.i45.17029
Pun M, Khazanov N, Galsurker O et al (2021) Phloretin, an apple phytoalexin, affects the virulence and fitness of pectobacterium brasiliense by interfering with quorum-sensing, front. Plant Sci. https://doi.org/10.3389/fpls.2021.671807
Gumul D, Ziobro R, Korus J, Kruczek M (2021) Apple pomace as a source of bioactive polyphenol compounds in gluten-free breads. Antioxidants (Basel, Switzerland) 10:807. https://doi.org/10.3390/antiox10050807
Chauhan A, Jang M, Kim Y (2020) Phloretin protects macrophages from E. coli-Induced inflammation through the TLR4 signaling pathway. J Microbiol Biotechnol 30:333–340. https://doi.org/10.4014/jmb.1910.10063
Wei LN, Shi CZ, Luo CX et al (2020) Phloretin inhibits biofilm formation by affecting quorum sensing under different temperature. LWT. https://doi.org/10.1016/j.lwt.2020.109668
Barreca D, Bellocco E, Laganà G et al (2014) Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem 160:292–297. https://doi.org/10.1016/j.foodchem.2014.03.118
Jeon D, Jeong M-C, Jnawali HN et al (2017) Phloretin exerts anti-tuberculosis activity and suppresses lung inflammation. Molecules 22:183. https://doi.org/10.3390/molecules22010183
Liu Y, Fan C, Pu L et al (2016) Phloretin induces cell cycle arrest and apoptosis of human glioblastoma cells through the generation of reactive oxygen species. J Neurooncol 128:217–223. https://doi.org/10.1007/s11060-016-2107-z
Zhou M, Zheng J, Bi J et al (2018) Synergistic inhibition of colon cancer cell growth by a combination of atorvastatin and phloretin. Oncol Lett 15:1985–1992. https://doi.org/10.3892/ol.2017.7480
Mariadoss AVA, Vinyagam R, Rajamanickam V et al (2019) Pharmacological aspects and potential use of phloretin: a systemic review. Mini-Reviews Med Chem 19:1060–1067. https://doi.org/10.2174/1389557519666190311154425
Wu C-H, Ho Y-S, Tsai C-Y et al (2009) In vitroandin vivostudy of phloretin-induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int J Cancer 124:2210–2219. https://doi.org/10.1002/ijc.24189
Wang Y, Li D, Lin H et al (2020) Enhanced oral bioavailability and bioefficacy of phloretin using mixed polymeric modified self-nanoemulsions. Food Sci Nutr 8:3545–3558. https://doi.org/10.1002/fsn3.1637
Cheng X, Xu X, Chen D et al (2019) Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed & Pharmacother 110:473–481. https://doi.org/10.1016/j.biopha.2018.11.082
Tejeda-Muñoz N, Albrecht LV, Bui MH, De Robertis EM (2019) Wnt canonical pathway activates macropinocytosis and lysosomal degradation of extracellular proteins. Proc Natl Acad Sci U S A 116:10402–10411. https://doi.org/10.1073/pnas.1903506116
Hosseini SS, Goudarzi H, Ghalavand Z et al (2020) Anti-proliferative effects of cell wall, cytoplasmic extract of Lactococcus lactis and nisin through down-regulation of cyclin D1 on SW480 colorectal cancer cell line. Iran J Microbiol. https://doi.org/10.18502/ijm.v12i5.4603
Abalos NN, Ebajo VD Jr, Camacho DH, Jacinto SD (2021) Cytotoxic and apoptotic activity of aglaforbesin derivative isolated from Aglaia loheri Merr. on HCT116 human colorectal cancer cells. Asian Pac J Cancer Prev. https://doi.org/10.31557/APJCP.2021.22.1.53
Nichol RJO, Khalaf AI, Sooda K et al (2019) Selective in vitro anti-cancer activity of non-alkylating minor groove binders. Medchemcomm 10:1620–1634. https://doi.org/10.1039/c9md00268e
Razak NA, Akhtar MN, Abu N et al (2017) The in vivo anti-tumor effect of curcumin derivative (2E,6E)-2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone (BHMC) on 4T1 breast cancer cells. RSC Adv 7:36185–36192. https://doi.org/10.1039/c7ra06580a
Dou J, Wang Z, Ma L et al (2018) Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget. https://doi.org/10.18632/oncotarget.24015
Dangroo NA, Singh J, Rath SK et al (2017) A convergent synthesis of novel alkyne–azide cycloaddition congeners of betulinic acid as potent cytotoxic agent. Steroids 123:1–12. https://doi.org/10.1016/j.steroids.2017.04.002
Sharma S, Chhimwal J, Kumar S et al (2021) Cytotoxic steroidal saponins from Polygonatum verticillatum Linn. Phytochem Lett 45:30–36. https://doi.org/10.1016/j.phytol.2021.07.010
Soni S, Saroch MK, Chander B et al (2019) MAPKAPK2 plays a crucial role in the progression of head and neck squamous cell carcinoma by regulating transcript stability. J Exp Clin Cancer Res 38:175. https://doi.org/10.1186/s13046-019-1167-2
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Zhang H, Yi JK, Huang H et al (2021) Rhein suppresses colorectal cancer cell growth by inhibiting the mtor pathway in vitro and in vivo. Cancers (Basel) 13:2176. https://doi.org/10.3390/cancers13092176
Zhang X-F, Sun R-Q, Jia Y-F et al (2016) Synthesis and mechanisms of action of novel harmine derivatives as potential antitumor agents. Sci Rep 6:33204. https://doi.org/10.1038/srep33204
Soumya T, Lakshmipriya T, Klika KD et al (2021) Anticancer potential of rhizome extract and a labdane diterpenoid from Curcuma mutabilis plant endemic to Western Ghats of India. Sci Rep 11:552. https://doi.org/10.1038/s41598-020-79414-8
Popov BV, Serikov VB, Petrov NS et al (2007) Lung epithelial cells induce endodermal differentiation in mouse mesenchymal bone marrow stem cells by paracrine mechanism. Tissue Eng 13:2441–2450. https://doi.org/10.1089/ten.2007.0001
Abu-Baker A, Laganiere J, Gaudet R et al (2013) Lithium chloride attenuates cell death in oculopharyngeal muscular dystrophy by perturbing Wnt/β-catenin pathway. Cell Death Dis 4:e821–e821. https://doi.org/10.1038/cddis.2013.342
Patil S, Kuman MM, Palvai S et al (2018) Impairing powerhouse in colon cancer cells by hydrazide-hydrazone-based small molecule. ACS Omega 3:1470–1481. https://doi.org/10.1021/acsomega.7b01512
Or C-HR, Huang C-W, Chang C-C et al (2020) Obatoclax, a Pan-BCL-2 inhibitor, downregulates Survivin to Induce apoptosis in human colorectal carcinoma cells via suppressing wnt/β-catenin signaling. Int J Mol Sci 21:1773. https://doi.org/10.3390/ijms21051773
Rennoll SA, Konsavage WM Jr, Yochum GS (2014) Nuclear AXIN2 represses MYC gene expression. Biochem Biophys Res Commun 443:217–222. https://doi.org/10.1016/j.bbrc.2013.11.089
Shang S, Hua F, Hu Z-W (2017) The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget. https://doi.org/10.18632/oncotarget.15687
Ahmad R, Singh JK, Wunnava A et al (2021) Emerging trends in colorectal cancer: dysregulated signaling pathways (Review). Int J Mol Med 47:14. https://doi.org/10.3892/ijmm.2021.4847
Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J (2020) Flavonoids as anticancer agents. Nutrients 12:457. https://doi.org/10.3390/nu12020457
Rodríguez-García C, Sánchez-Quesada C, Gaforio JJ (2019) Dietary flavonoids as cancer chemopreventive agents: an updated review of human studies. Antioxidants (Basel, Switzerland) 8:137. https://doi.org/10.3390/antiox8050137
Perez-Vizcaino F, Fraga CG (2018) Research trends in flavonoids and health. Arch Biochem Biophys 646:107–112. https://doi.org/10.1016/j.abb.2018.03.022
Abotaleb M, Samuel SM, Varghese E et al (2018) Flavonoids in cancer and apoptosis. Cancers (Basel) 11:28. https://doi.org/10.3390/cancers11010028
Hadi SM, Asad SF, Singh S, Ahmad A (2000) Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life (International Union Biochem Mol Biol Life) 50(167):171. https://doi.org/10.1080/152165400300001471
Liu X, Zhang Y, Wu S et al (2020) Palmatine induces G2/M phase arrest and mitochondrial-associated pathway apoptosis in colon cancer cells by targeting AURKA. Biochem Pharmacol. https://doi.org/10.1016/j.bcp.2020.113933
Jing Y, Yang J, Wang Y et al (2006) Alteration of subcellular redox equilibrium and the consequent oxidative modification of nuclear factor κB are critical for anticancer cytotoxicity by emodin, a reactive oxygen species-producing agent. Free Radic Biol Med 40:2183–2197. https://doi.org/10.1016/j.freeradbiomed.2006.02.016
Lebedeva IV, Washington I, Sarkar D et al (2007) Strategy for reversing resistance to a single anticancer agent in human prostate and pancreatic carcinomas. Proc Natl Acad Sci U S A 104:3484–3489. https://doi.org/10.1073/pnas.0700042104
Mitchell C, Park MA, Zhang G et al (2007) 17-Allylamino-17-demethoxygeldanamycin enhances the lethality of deoxycholic acid in primary rodent hepatocytes and established cell lines. Mol Cancer Ther 6:618–632. https://doi.org/10.1158/1535-7163.mct-06-0532
Yasuda S, Yogosawa S, Izutani Y et al (2009) Cucurbitacin B induces G2 arrest and apoptosis via a reactive oxygen species-dependent mechanism in human colon adenocarcinoma SW480 cells. Mol Nutr & Food Res 54:559–565. https://doi.org/10.1002/mnfr.200900165
Kim E-K, Cho JH, Kim E, Kim YJ (2017) Ursodeoxycholic acid inhibits the proliferation of colon cancer cells by regulating oxidative stress and cancer stem-like cell growth. PLoS ONE 12:e0181183–e0181183. https://doi.org/10.1371/journal.pone.0181183
Pfeffer CM, Singh ATK (2018) Apoptosis: a target for anticancer therapy. Int J Mol Sci 19:448. https://doi.org/10.3390/ijms19020448
Ji H (2018) Hot spot-based design of small-molecule inhibitors for protein-protein interactions. In: Sheng C, Georg GI (eds) Targeting Protein-Protein Interactions by Small Molecules. Springer, Singapore, pp 53–71
Jensen M, García-Bonete M-J, Anindya AL, et al (2022) Survivin prevents the Polycomb Repressor Complex 2 from methylating Histone 3 lysine 27
Bu H, Liu D, Cui J et al (2019) Wnt/β-catenin signaling pathway is involved in induction of apoptosis by oridonin in colon cancer COLO205 cells. Transl Cancer Res. https://doi.org/10.21037/tcr.2019.08.25
Zhou Y, Li X, Ye M (2021) Morusin inhibits the growth of human colorectal cancer HCT116-derived sphere-forming cells via the inactivation of Akt pathway. Int J Mol Med 47:1. https://doi.org/10.3892/ijmm.2021.4884
Kim U, Kim C-Y, Lee JM et al (2019) Phloretin inhibits the human prostate cancer cells through the generation of reactive oxygen species. Pathol & Oncol Res 26:977–984. https://doi.org/10.1007/s12253-019-00643-y
Zhang S, Guo S, Li Z et al (2019) High expression of HSP90 is associated with poor prognosis in patients with colorectal cancer. PeerJ 7:e7946–e7946. https://doi.org/10.7717/peerj.7946
Huang X-H, Yan X, Zhang Q-H et al (2020) Direct targeting of HSP90 with daurisoline destabilizes β-catenin to suppress lung cancer tumorigenesis. Cancer Lett 489:66–78. https://doi.org/10.1016/j.canlet.2020.05.024
Kurashina R, Ohyashiki JH, Kobayashi C et al (2009) Anti-proliferative activity of heat shock protein (Hsp) 90 inhibitors via β-catenin/TCF7L2 pathway in adult T cell leukemia cells. Cancer Lett 284:62–70. https://doi.org/10.1016/j.canlet.2009.04.012
Shtutman M, Zhurinsky J, Simcha I et al (1999) The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A 96:5522–5527. https://doi.org/10.1073/pnas.96.10.5522
Li Y-J, Wei Z-M, Meng Y-X, Ji X-R (2005) Beta-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: relationships with carcinogenesis and metastasis. World J Gastroenterol 11:2117–2123. https://doi.org/10.3748/wjg.v11.i14.2117
Kaur M, Velmurugan B, Tyagi A et al (2010) Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of beta-catenin-dependent signaling. Neoplasia 12:415–424. https://doi.org/10.1593/neo.10188
Yamaki H, Nakajima M, Shimotohno KW, Tanaka N (2011) Molecular basis for the actions of Hsp90 inhibitors and cancer therapy. J Antibiot (Tokyo) 64:635–644. https://doi.org/10.1038/ja.2011.60
Tao L, Gu Y, Zheng J et al (2019) Weichang’an suppressed migration and invasion of HCT116 cells by inhibiting Wnt/β-catenin pathway while upregulating ARHGAP25. Biotechnol Appl Biochem 66:787–793. https://doi.org/10.1002/bab.1784
Kaufmann L, Marinescu G, Nazarenko I et al (2011) LiCl induces TNF-α and FasL production, thereby stimulating apoptosis in cancer cells. Cell Commun Signal 9:15. https://doi.org/10.1186/1478-811X-9-15
Gu L, Sun R, Wang W, Xia Q (2022) Nanostructured lipid carriers for the encapsulation of phloretin: preparation and in vitro characterization studies. Chem Phys Lipids. https://doi.org/10.1016/j.chemphyslip.2021.105150
Acknowledgements
The authors are highly thankful to the Director, CSIR-Institute of Himalayan Bioresource Technology (CSIR-IHBT), Palampur, India for his support and providing all the necessary facilities. SK is highly grateful to the Indian Council of Medical Research, New Delhi for providing the ICMR-JRF fellowship and to the Academy of Scientific and Innovative Research (AcSIR), Ghaziabad for Ph.D. registration. CSIR-IHBT communication number for this manuscript is 5103.
Funding
This study was supported by grants from the CSIR project MLP 0155 and MLP0204.
Author information
Authors and Affiliations
Contributions
SK: Conceptualization, Methodology, Validation, Formal analysis, writing original draft. YSP: Conceptualization, Resources, Review and editing, Investigation, Supervision, Funding acquisition. Both authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kapoor, S., Padwad, Y.S. Phloretin induces G2/M arrest and apoptosis by suppressing the β-catenin signaling pathway in colorectal carcinoma cells. Apoptosis 28, 810–829 (2023). https://doi.org/10.1007/s10495-023-01826-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01826-4