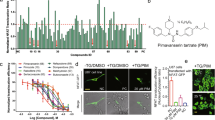
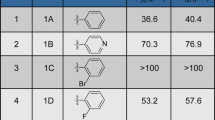
Abstract
Glioblastoma remains the most malignant of all primary adult brain tumours with poor patient survival and limited treatment options. This study adopts a drug repurposing approach by investigating the anti-cancer activity of a derivative of the antipsychotic drug phenothiazine (DS00329) in malignant U251 and U87 glioblastoma cells. Results from MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and clonogenic assays showed that DS00329 inhibited short-term glioblastoma cell viability and long-term survival while sparing non-cancerous cells. Western blot analysis with an antibody to γH2AX showed that DS00329 induced DNA damage and flow cytometry and western blotting confirmed that it triggered a G1 cell cycle arrest which correlated with decreased levels in Cyclin A, Cyclin B, Cyclin D1 and cyclin dependent kinase 2 and an increase in levels of the cyclin dependent kinase inhibitor p21. DS00329 treated glioblastoma cells exhibited morphological and molecular markers typical of apoptotic cells such as membrane blebbing and cell shrinkage and an increase in levels of cleaved PARP. Flow cytometry with annexin V-FITC/propidium iodide staining confirmed that DS00329 induced apoptotic cell death in glioblastoma cells. We also show that DS00329 treatment of glioblastoma cells led to an increase in the autophagosome marker LC3-II and autophagy inhibition studies using bafilomycin A1 and wortmannin, showed that DS00329-induced-autophagy was a pro-death mechanism. Furthermore, DS00329 treatment of glioblastoma cells inhibited the phosphatidylinositol 3′-kinase/Akt cell survival pathway. Our findings suggest that DS00329 may be an effective treatment for glioblastoma and provide a rationale for further exploration and validation of the use of phenothiazines and their derivatives in the treatment of glioblastoma.








Similar content being viewed by others
Abbreviations
- PTZ:
-
Phenothiazine
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- γH2AX:
-
Phosphorylated H2A histone family member X
- CDK2:
-
Cyclin-dependent kinase 2
- PARP:
-
Poly (ADP-ribose) polymerase
- FITC:
-
Fluorescein isothiocyanate (FITC)
- LC3:
-
Microtubule-associated protein light chain 3
- FACS:
-
Fluorescence-activated cell sorting
- AVO:
-
Acidic vesicular organelles
- PI3K/Akt:
-
Phosphatidylinositol 3′ kinase/Akt
- MAPK:
-
Mitogen activated protein kinase
- ATM:
-
Ataxia-telangiectasia mutated
References
Lin Y-J, Chiu H-Y, Chiou M-J, Huang Y-C, Wei K-C, Kuo C-F, Hsu J-T, Chen P-Y (2017) Trends in the incidence of primary malignant brain tumors in Taiwan and correlation with comorbidities: a population-based study. Clin Neurol Neurosurg 159:72–82
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359(5):492–507
Ohgaki H (2009) Epidemiology of brain tumors. Cancer Epidemiol 25:323–342
Mellinghoff IK, Gilbertson RJ (2017) Brain tumors: challenges and opportunities to cure. J Clin Oncol 35(21):2343–2345
Elliott PJ, Hayward NJ, Huff MR, Nagle TL, Black KL, Bartus RT (1996) Unlocking the blood–brain barrier: a role for RMP-7 in brain tumor therapy. Exp Neurol 141(2):214–224
Giese A, Bjerkvig R, Berens M, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21(8):1624–1636
Smith JS, Jenkins RB (2000) Genetic alterations in adult diffuse glioma: occurrence, significance, and prognostic implications. Front Biosci 5(1):213–231
Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G (2007) Long-term survival with glioblastoma multiforme. Brain 130(10):2596–2606
Oprea TI, Bauman JE, Bologa CG, Buranda T, Chigaev A, Edwards BS, Jarvik JW, Gresham HD, Haynes MK, Hjelle B (2011) Drug repurposing from an academic perspective. Drug Discov Today 8(3–4):61–69
Ashburn TT, Thor KB (2004) Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 3(8):673
Gupta R (1988) Bioactive molecules: phenothiazine and 1, 4-benzothiazines, Chemical and Biological Aspects, vol 4. Elsevier, Amsterdam
Morak-Młodawska B, Pluta K, Matralis AN, Kourounakis AP (2010) Antioxidant Activity of Newly Synthesized 2, 7-Diazaphenothiazines. Arch Pharm 343(5):268–273
Kang S, Dong SM, Kim B-R, Park MS, Trink B, Byun H-J, Rho SB (2012) Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells. Apoptosis 17(9):989–997
Cheng H-W, Liang Y-H, Kuo Y-L, Chuu C-P, Lin C-Y, Lee M-H, Wu AT, Yeh C-T, Chen EI, Whang-Peng J (2015) Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Dis 6(5):e1753
Yong M, Yu T, Tian S, Liu S, Xu J, Hu J, Hu L (2017) DR2 blocker thioridazine: A promising drug for ovarian cancer therapy. Oncol Lett 14(6):8171–8177
Seervi M, Rani A, Sharma AK, Santhosh Kumar TR (2018) ROS mediated ER stress induces Bax-Bak dependent and independent apoptosis in response to Thioridazine. Biomed Pharmacother 106:200–209
Wu C-H, Bai L-Y, Tsai M-H, Chu P-C, Chiu C-F, Chen MY, Chiu S-J, Chiang J-H, Weng J-R (2016) Pharmacological exploitation of the phenothiazine antipsychotics to develop novel antitumor agents–a drug repurposing strategy. Sci Rep 6:27540
Brem B, Gal E, Găină L, Silaghi-Dumitrescu L, Fischer-Fodor E, Tomuleasa CI, Grozav A, Zaharia V, Filip L, Cristea C (2017) Novel thiazolo [5, 4-b] phenothiazine derivatives: synthesis, structural characterization, and in vitro evaluation of antiproliferative activity against human leukaemia. Int J Mol Sci 18(7):1365
Ghorab MM, Alsaid MS, Samir N, Abdel-Latif GA, Soliman AM, Ragab FA, El Ella DAA (2017) Aromatase inhibitors and apoptotic inducers: Design, synthesis, anticancer activity and molecular modeling studies of novel phenothiazine derivatives carrying sulfonamide moiety as hybrid molecules. Eur J Med Chem 134:304–315
Ma X-H, Liu N, Lu J-L, Zhao J, Zhang X-J (2017) Design, synthesis and antiproliferative activity of novel phenothiazine-1, 2, 3-triazole analogues. J Chem Res 41(12):696–698
Dillon CP, Green DR (2016) Molecular cell biology of apoptosis and necroptosis in cancer. In: Apoptosis in cancer pathogenesis and anti-cancer therapy. Springer, New York, pp 1–23
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1(2):112
Wong RS (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30(1):87
Hongmei Z (2012) Extrinsic and intrinsic apoptosis signal pathway review. In: Apoptosis and Medicine. Intech
Mizushima N, Klionsky DJ (2007) Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr 27:19–40
Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, Vicinanza M, Menzies FM, Rubinsztein DC (2016) Mammalian autophagy: how does it work? Annu Rev Biochem 85:685–713
Cheng S-Y, Chen N-F, Kuo H-M, Yang S-N, Sung C-S, Sung P-J, Wen Z-H, Chen W-F (2018) Prodigiosin stimulates endoplasmic reticulum stress and induces autophagic cell death in glioblastoma cells. Apoptosis 23(5):314–328
Wu B, Tan M, Cai W, Wang B, He P, Zhang X (2018) Arsenic trioxide induces autophagic cell death in osteosarcoma cells via the ROS-TFEB signaling pathway. Biochem Biophys Res Commun 496(1):67–175
Ni Y, Wu S, Wang X, Zhu G, Chen X, Ding Y, Jiang W (2018) Cucurbitacin I induces pro-death autophagy in A549 cells via the ERK-mTOR-STAT3 signaling pathway. J Cell Biochem 119(7):6104–6112
Del Bello B, Toscano M, Moretti D, Maellaro E (2013) Cisplatin-induced apoptosis inhibits autophagy, which acts as a pro-survival mechanism in human melanoma cells. PLoS ONE 8(2):e57236
Guzmán C, Bagga M, Kaur A, Westermarck J, Abankwa D (2014) Colonyarea: an imagej plugin to automatically quantify colony formation in clonogenic assays. PLoS ONE 9(3):e92444
Franken NA, Rodermond HM, Stap J, Haveman J, Van Bree C (2006) Clonogenic assay of cells in vitro. Nat Protoc 1(5):2315
Torsvik A, Stieber D, Enger PØ, Golebiewska A, Molven A, Svendsen A, Westermark B, Niclou SP, Olsen TK, Chekenya Enger M (2014) U-251 revisited: genetic drift and phenotypic consequences of long-term cultures of glioblastoma cells. Cancer Med 3(4):812–824
Gangopadhyay S, Karmakar P, Dasgupta U, Chakraborty A (2007) Trifluoperazine stimulates ionizing radiation induced cell killing through inhibition of DNA repair. Mutat Res Genet Toxicol Environ Mutagen 633(2):117–125
Zong D, Hååg P, Yakymovych I, Lewensohn R, Viktorsson K (2011) Chemosensitization by phenothiazines in human lung cancer cells: impaired resolution of γH2AX and increased oxidative stress elicit apoptosis associated with lysosomal expansion and intense vacuolation. Cell Death Dis 2(7):e181
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273(10):5858–5868
Martin OA, Bonner WM (2006) γ-H2AX in cancer cells: a potential biomarker for cancer diagnostics, prediction and recurrence. Cell Cycle 5(24):2909–2913
Brázdová M, Quante T, Tögel L, Walter K, Loscher C, Tichý V, Činčárová L, Deppert W, Tolstonog GV (2009) Modulation of gene expression in U251 glioblastoma cells by binding of mutant p53 R273H to intronic and intergenic sequences. Nucleic Acids Res 37(5):1486–1500
Luan S, Sun L, Huang F (2010) MicroRNA-34a: a novel tumor suppressor in p53-mutant glioma cell line U251. Arch Med Res 41(2):67–74
Fadeel B, Orrenius S (2005) Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med 258(6):479–517
Häcker G (2000) The morphology of apoptosis. Cell Tissue Res 301(1):5–17
Simbulan-Rosenthal CM, Rosenthal DS, Iyer S, Boulares AH, Smulson ME (1998) Transient poly (ADP-ribosyl) ation of nuclear proteins and role of poly (ADP-ribose) polymerase in the early stages of apoptosis. J Biol Chem 273(22):13703–13712
Crowley LC, Marfell BJ, Scott AP, Waterhouse NJ (2016) Quantitation of apoptosis and necrosis by annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb Protoc. https://doi.org/10.1101/pdb.prot087288
Liu Y, Lu J, Zhang Z, Zhu L, Dong S, Guo G, Li R, Nan Y, Yu K, Zhong Y (2017) Amlexanox, a selective inhibitor of IKBKE, generates anti-tumoral effects by disrupting the Hippo pathway in human glioblastoma cell lines. Cell Death Dis 8(8):e3022
Miao J, Jiang Y, Wang D, Zhou J, Fan C, Jiao F, Liu B, Zhang J, Wang Y, Zhang Q (2015) Trichosanthin suppresses the proliferation of glioma cells by inhibiting LGR5 expression and the Wnt/β-catenin signaling pathway. Oncol Rep 34(6):2845–2852
Aliwaini S, Swarts AJ, Blanckenberg A, Mapolie S, Prince S (2013) A novel binuclear palladacycle complex inhibits melanoma growth in vitro and in vivo through apoptosis and autophagy. Biochem Pharmacol 86(12):1650–1663
Mathew R, Karantza-Wadsworth V, White E (2007) Role of autophagy in cancer. Nat Rev Cancer 7(12):961
Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J (2001) A Novel Response of Cancer Cells to Radiation Involves Autophagy and Formation of Acidic Vesicles. Cancer Res 61(2):439–444
Tanida I, Ueno T, Kominami E (2004) LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36(12):2503–2518
Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y (1998) Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 23(1):33–42
Ma K, Zhang C, Huang M-Y, Li W-Y, Hu G-Q (2016) Cinobufagin induces autophagy-mediated cell death in human osteosarcoma U2OS cells through the ROS/JNK/p38 signaling pathway. Oncol Rep 36(1):90–98
Shin SY, Lee KS, Choi Y-K, Lim HJ, Lee HG, Lim Y, Lee YH (2013) The antipsychotic agent chlorpromazine induces autophagic cell death by inhibiting the Akt/mTOR pathway in human U-87MG glioma cells. Carcinogenesis 34(9):2080–2089
Purow B (2016) Repurposing existing agents as adjunct therapies for glioblastoma. Neuro-Oncol Pract 3(3):154–163. https://doi.org/10.1093/nop/npv041
Abbruzzese C, Matteoni S, Signore M, Cardone L, Nath K, Glickson JD, Paggi MG (2017) Drug repurposing for the treatment of glioblastoma multiforme. J Exp Clin Cancer Res 36(1):169
Basso J, Miranda A, Sousa J, Pais A, Vitorino C (2018) Repurposing drugs for glioblastoma: from bench to bedside. Cancer Lett 428:173–183
Tan SK, Jermakowicz A, Mookhtiar AK, Nemeroff CB, Schürer SC, Ayad NG (2018) Drug repositioning in glioblastoma: a pathway perspective. Front Pharmacol 9:218
Kendall RT, Feghali-Bostwick CA (2014) Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol 5:123
Fiebig H, Maier A, Burger A (2004) Clonogenic assay with established human tumour xenografts: correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur J Cancer 40(6):802–820
Pessina A, Malerba I, Gribaldo L (2005) Hematotoxicity testing by cell clonogenic assay in drug development and preclinical trials. Curr Pharm Des 11(8):1055–1065
Hait WN, Morris S, Lazo JS, Figlin RJ, Durivage HJ, White K, Schwartz PE (1989) Phase I trial of combined therapy with bleomycin and the calmodulin antagonist, trifluoperazine. Cancer Chemother Pharmacol 23(6):358–362
Sridhar K, Krishan A, Samy T, Sauerteig A, Wellham L, McPhee G, Duncan R, Anac S, Ardalan B, Benedetto P (1993) Prochlorperazine as a doxorubicin-efflux blocker: phase I clinical and pharmacokinetics studies. Cancer Chemother Pharmacol 31(6):423–430
Annovazzi L, Mellai M, Schiffer D (2017) Chemotherapeutic Drugs: DNA Damage and Repair in Glioblastoma. Cancers 9(6):57
Kastan MB, Bartek J (2004) Cell-cycle checkpoints and cancer. Nature 432(7015):316
Maréchal A, Zou L (2013) DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol 5(9):a012716
Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ (2002) Genomic instability in mice lacking histone H2AX. Science 296(5569):922–927
Lukas C, Falck J, Bartkova J, Bartek J, Lukas J (2003) Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat Cell Biol 5(3):255
Buscemi G, Perego P, Carenini N, Nakanishi M, Chessa L, Chen J, Khanna K, Delia D (2004) Activation of ATM and Chk2 kinases in relation to the amount of DNA strand breaks. Oncogene 23(46):7691
Lowe SW, Lin AW (2000) Apoptosis in cancer. Carcinogenesis 21(3):485–495. https://doi.org/10.1093/carcin/21.3.485
Torigoe T, Izumi H, Ishiguchi H, Yoshida Y, Tanabe M, Yoshida T, Igarashi T, Niina I, Wakasugi T, Imaizumi T (2005) Cisplatin resistance and transcription factors. Curr Med Chem 5(1):15–27
Florea A-M, Büsselberg D (2011) Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers 3(1):1351–1371
Clarke PG (2002) Apoptosis: from morphological types of cell death to interacting pathways. Trends Pharmacol Sci 23(7):308–309
Gozuacik D, Kimchi A (2007) Autophagy and cell death. Curr Top Dev Biol 78:217–245
Laubenbacher R, Hower V, Jarrah A, Torti SV, Shulaev V, Mendes P, Torti FM, Akman S (2009) A systems biology view of cancer. Biochimica et Biophysica Acta BBA 1796(2):129–139
Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I (2003) Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res 63(9):2103–2108
Qian W, Liu J, Jin J, Ni W, Xu W (2007) Arsenic trioxide induces not only apoptosis but also autophagic cell death in leukemia cell lines via up-regulation of Beclin-1. Leuk Res 31(3):329–339
Fan Y, Chiu J-F, Liu J, Deng Y, Xu C, Zhang J, Li G (2018) Resveratrol induces autophagy-dependent apoptosis in HL-60 cells. BMC Cancer 18(1):581. https://doi.org/10.1186/s12885-018-4504-5
White E (2012) Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 12(6):401
Amaravadi R, Kimmelman AC, White E (2016) Recent insights into the function of autophagy in cancer. Genes Dev 30(17):1913–1930
Acknowledgements
This work was supported by the Cancer Association of South Africa (CANSA), the South Africa National Research Foundation (NRF), the South Africa Medical Research Council (SA MRC), the University of Cape Town, the University of the Western Cape and the University of Benin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Omoruyi, S.I., Ekpo, O.E., Semenya, D.M. et al. Exploitation of a novel phenothiazine derivative for its anti-cancer activities in malignant glioblastoma. Apoptosis 25, 261–274 (2020). https://doi.org/10.1007/s10495-020-01594-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-020-01594-5